Medical device sector is one of the fastest growing market sectors in Taiwan with the industry maintaining double-digit growth for over a decade. Hence, it presents excellent opportunities for foreign exporters of medical equipment and supplies. Qualtech is your ideal partner to for your foray into the Taiwan medical device market for an easy and smooth registration process.

Qualtech in Taiwan
Pass Several GCP Inspections
Taiwan have enacted Good Clinical Practice (GCP) since 2010. Qualtech has successfully assisted many clinical institutes to complete and pass GCP inspections ensuring the clinical performances.
Acquire Over 3000+ Approvals
Qualtech Taipei team consists of 90% Master and PhD personnel. With this high-quality and experienced team, we support clients to get Taiwan approvals efficiently.

Medical Device Registration
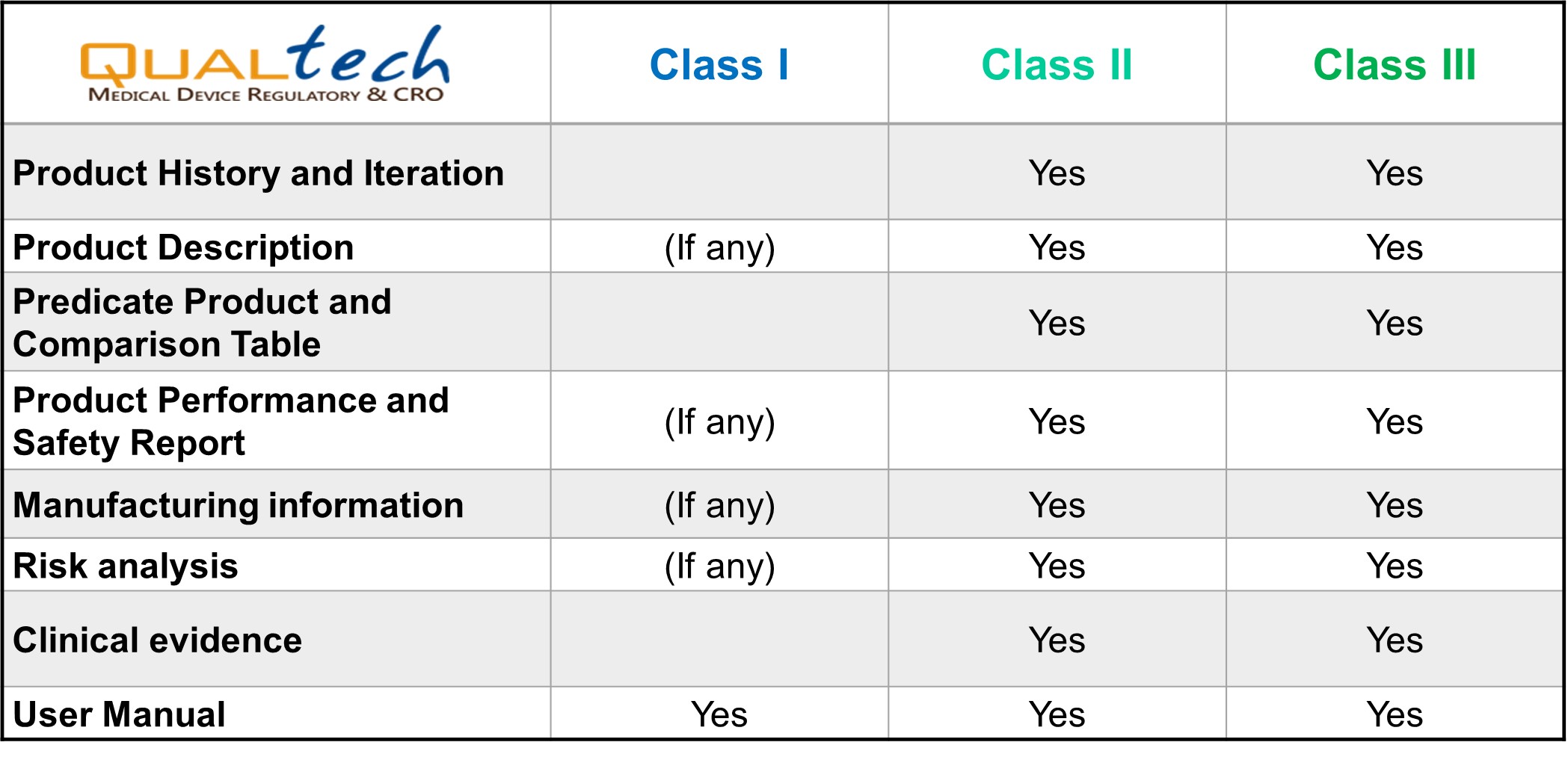
With more than a decade's experience in compiling successful TFDA submission dossiers, our in-house experts are able to provide you with excellent professional service in preparing customized dossier and complying with evaluators’ queries for medical device registration in Taipei.

License Holder
With changing and strict environment in Taipei, it’s important to find a stable and professional local agent to benefit your management of the Taiwan market. Qualtech owns an excellent license holder system to support you with the post-market surveillance, reimbursement, regulation compliance, license renewal and other license-related services.

Clinical Trial & CER Writing
There are two options for clinical evidence to TFDA: Conducting clinical trial or submitting CER complied with TFDA regulation. Qualtech has PhD-lead medical writing group, and an independent clinical trial team. Our experienced registration in medical device can ensure your clinical evidence meet TFDA’s requirement.
Overview of Taiwan Regulation
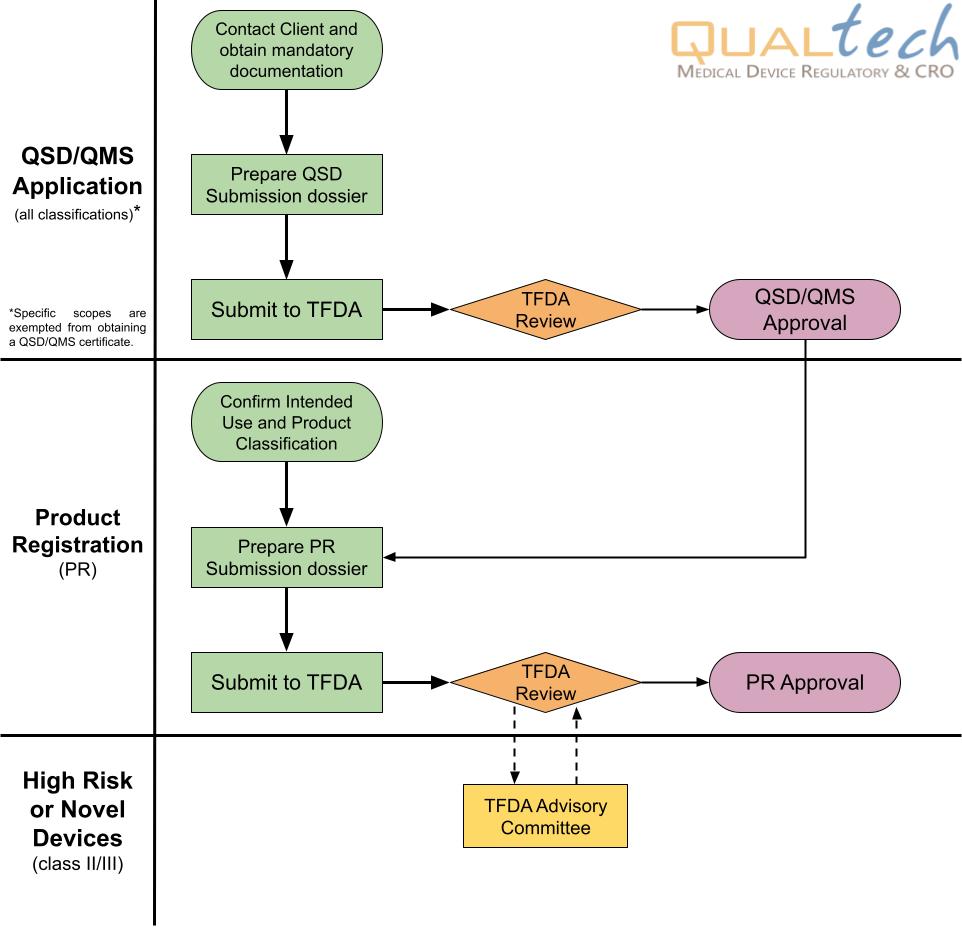
The registration of imported medical devices in Taiwan is a two-stage process, which is highly regulated by the Taiwan Food and Drug Administration (TFDA). The 1st stage is applying for applying for QSD/QMS certificate, a.k.a. the quality management system of each manufacturer, and the 2nd stage is applying for medical device registration. Foreign manufacturers must appoint an in-country License Holder (or local representative) to manage regulatory work of registered device. Furthermore, the license holder has capacity to authorize designated importer/distributor to process the market related activities, such as importation, reimbursement, bidding...
Note: Kindly see the Regulations Governing Issuance of Medical Device License, Listing and Annual Declaration enforced in 2021 for more details.
FDA Target Reviewing Evaluation Time
After successful internal evaluation of the submission dossier, our in-house experts will then submit it to TFDA and subject to thorough review. Below is a list of the evaluation times for different types of transactions involving QSD/QMS application and medical device registration.
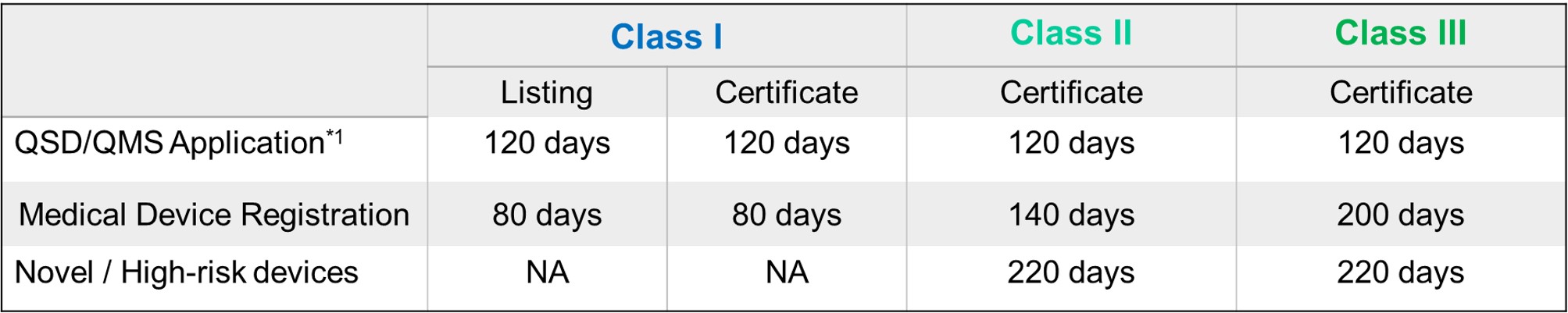
*1: Only specific class I medical devices are exempt from obtaining QSD/QMS approval. The list is periodically updated by TFDA.
*2: High-risk devices or novel devices may undergo TFDA advisory committee review. The turnaround time for the project will then be prolonged.
-
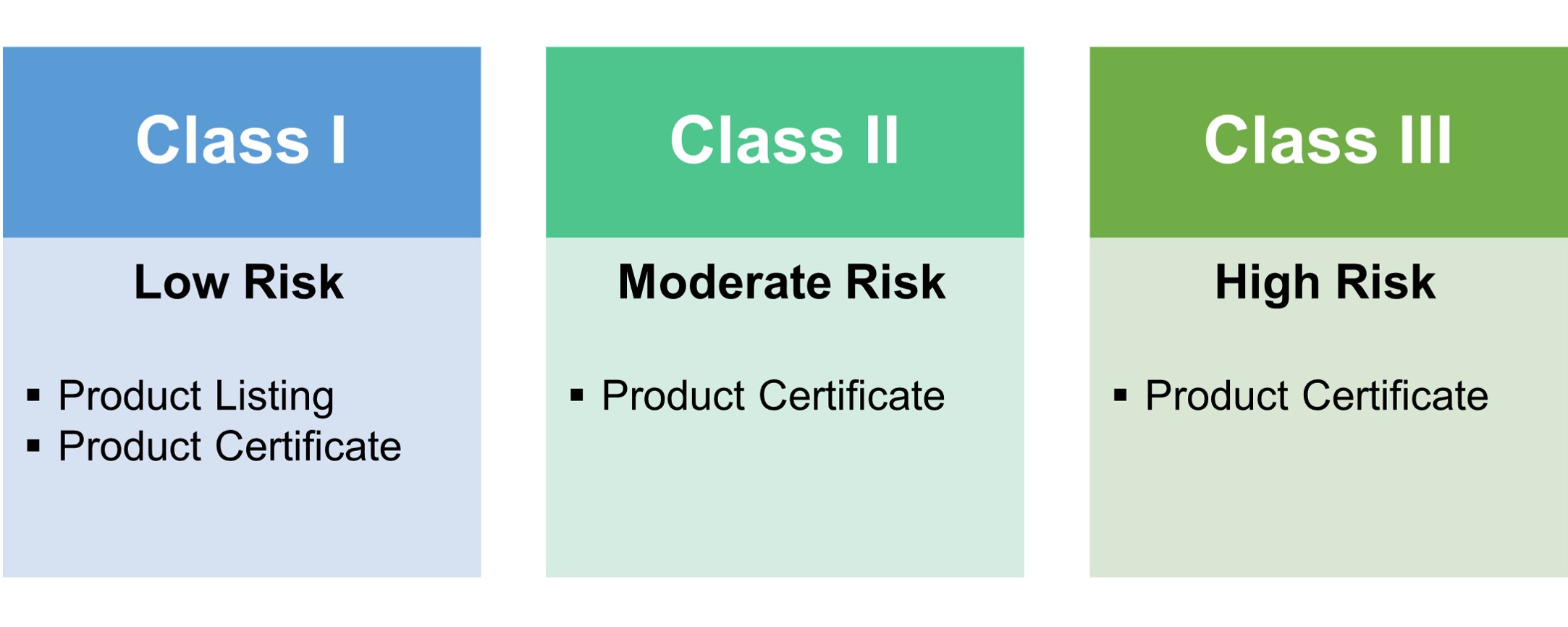
Types of Certificates: QSD Certificate, Product listing, Product Certificate
-
QSD Certificate: 3 years.
-
Product listing: Annual review (During October)
-
Product Certificate: 5 years
Quality System Documentation (QSD) is part of TFDA's Medical Device Quality Management System Regulation, to ensure the qualities of manufacturers before product registration.
Under TFDA regulation, only specific Class I medical devices are exempted from obtaining the QSD/QMS certificate. TFDA periodically updates the list of these specific scopes.
Other than that, QSD/QMS is essential for all medical devices. All the manufacturers need to obtain and maintain the QSD/QMS certificates for product registration and import.
For QSD application, TFDA provides several approaches that may benefit the manufacturers from the USA, Puerto Rico, Guam, European Union (EU), Switzerland, Lichtenstein, and Japan. Under specific circumstances, TFDA will acknowledge the audit or inspection reports from overseas authorities as substitute for the standard applications. Manufacturers from other regions will need to follow through standard or essential application mode.
For more information, please refer to the Taiwan FDA's official website or you may contact us for a free consultation.
我們透過Cookies蒐集您的瀏覽記錄,以了解您如何使用我們的網站,從而分析及改善您的體驗。如繼續使用我們的網站,即表示您接受我們使用 Cookies。