|
China Medical Device Registration |
|
Medical device sector is one of the fastest growing market sectors in China with the industry maintaining double-digit growth for over a decade. Hence, it presents excellent opportunities for foreign exporters of medical equipment and supplies. Qualtech is your ideal partner to for your foray into the China medical device market for an easy and smooth registration process. |

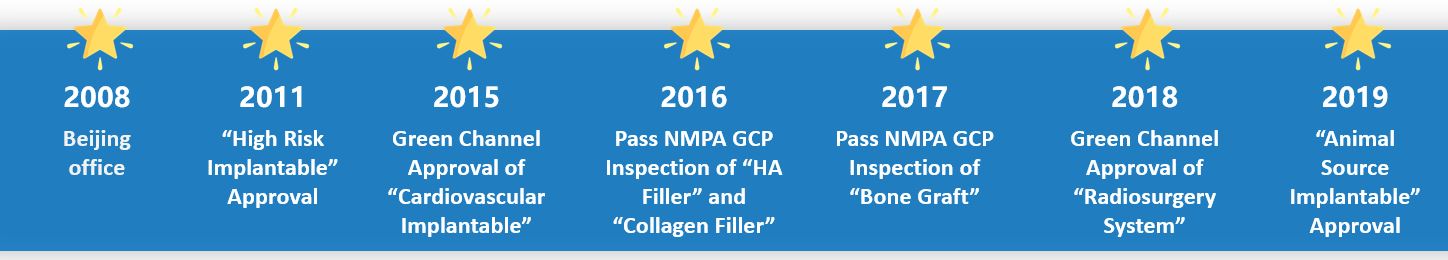
Qualtech in China
Pass Several GCP Inspections
China implements Good Clinical Practice from 2015. Qualtech has assisted many clinical sites to pass GCP inspection to ensure clinical performance.
Get Over 500 Approvals
Qualtech team is composed of 80% Master and PhD. With high-qualified and experienced team, we assists clients to get China approval in efficiency!

Medical Device Registration
With more than a decade’s experience in compiling successful NMPA (formerly CFDA) submission dossiers, our in-house experts are able to provide you with excellent professional service in preparing customized dossier and complying with evaluators’ queries for medical device registration in China.

Clinical Trial and CER Writing
There are two options for clinical evidence to NMPA: Conducting clinical trial or submitting clinical evaluation report (CER) complied with China regulation. Qualtech has PhD-led medical writing group, and an independent clinical trial team. Our experienced registration in medical device can ensure your clinical evidence meet NMPA’s requirement.

Local Agent
With changing and strict environment in China, it’s important to find a stable and professional local agent to assist you manage China market. Qualtech owns an excellent license holder system to help you easily manage the post-market surveillance, health insurance, regulation compliance, license renewal and other license-related works.

Product Testing
The NMPA requires in-country testing the Class II & III medical devices regarding China standards. Qualtech can provide a detailed assessment of the NMPA requirements for testing for your device and assist with registration.
| Overview of Registration in China | |
|
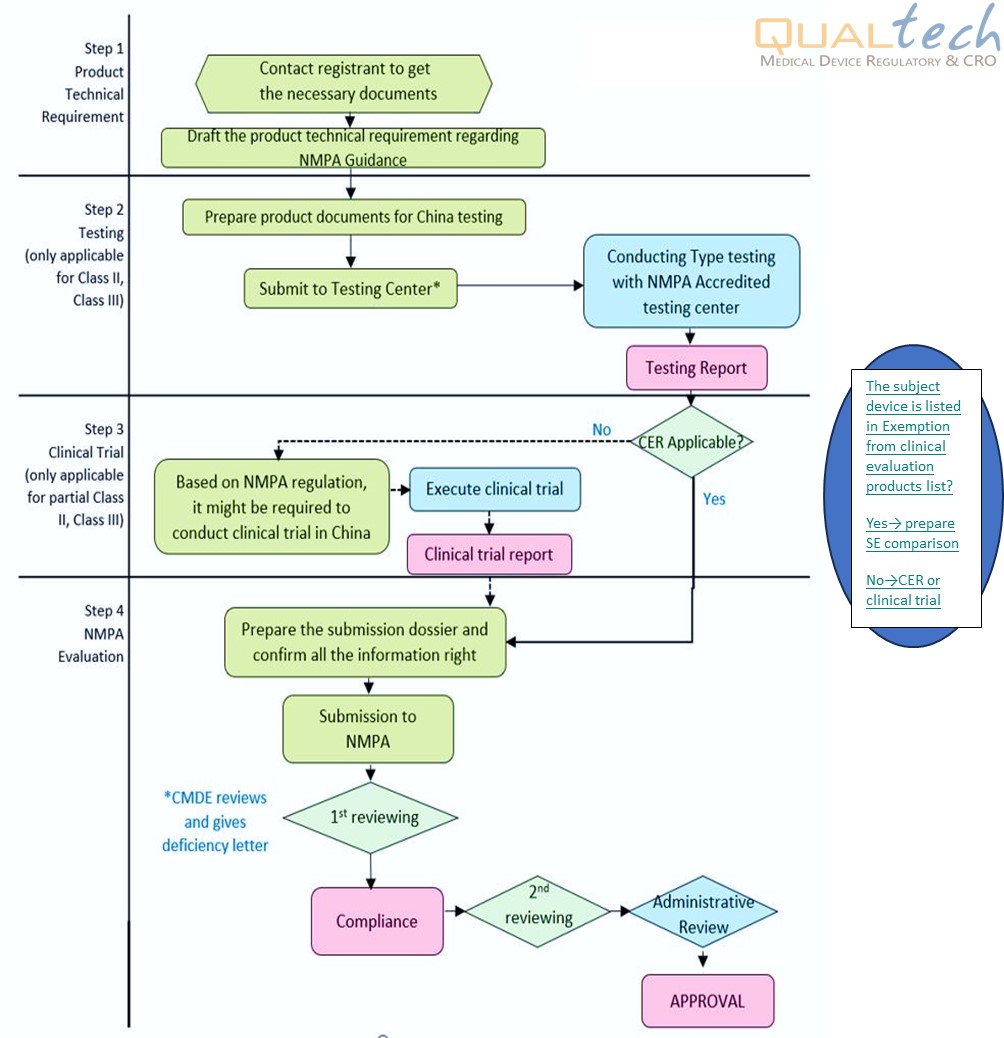
The registration of medical device in China is a laborious process. The process is highly regulated by the China National Medical Product Administration (NMPA). In the process, firstly, the product technical requirement must be prepared. Following that, the product must be tested at any one of the NMPA - Accredited test centers which include official or accredited third-party laboratories with China GB/YY Standard or China Guidance. When the test report is obtained, the registrant shall evaluate whether the subject device is listed in Exemption from Clinical Evaluation Medical Devices. If yes, the registrant shall do the SE comparison regarding China Guidance of Comparison Description for Products in Exemption List. Otherwise, the registrant shall do the clinical evaluation regarding China CER Guidance, if necessary, the registrant should conduct clinical trial in China. Once the clinical evaluation report (or clinical trial report) is presented, the product can be submitted for NMPA’s product registration.
|

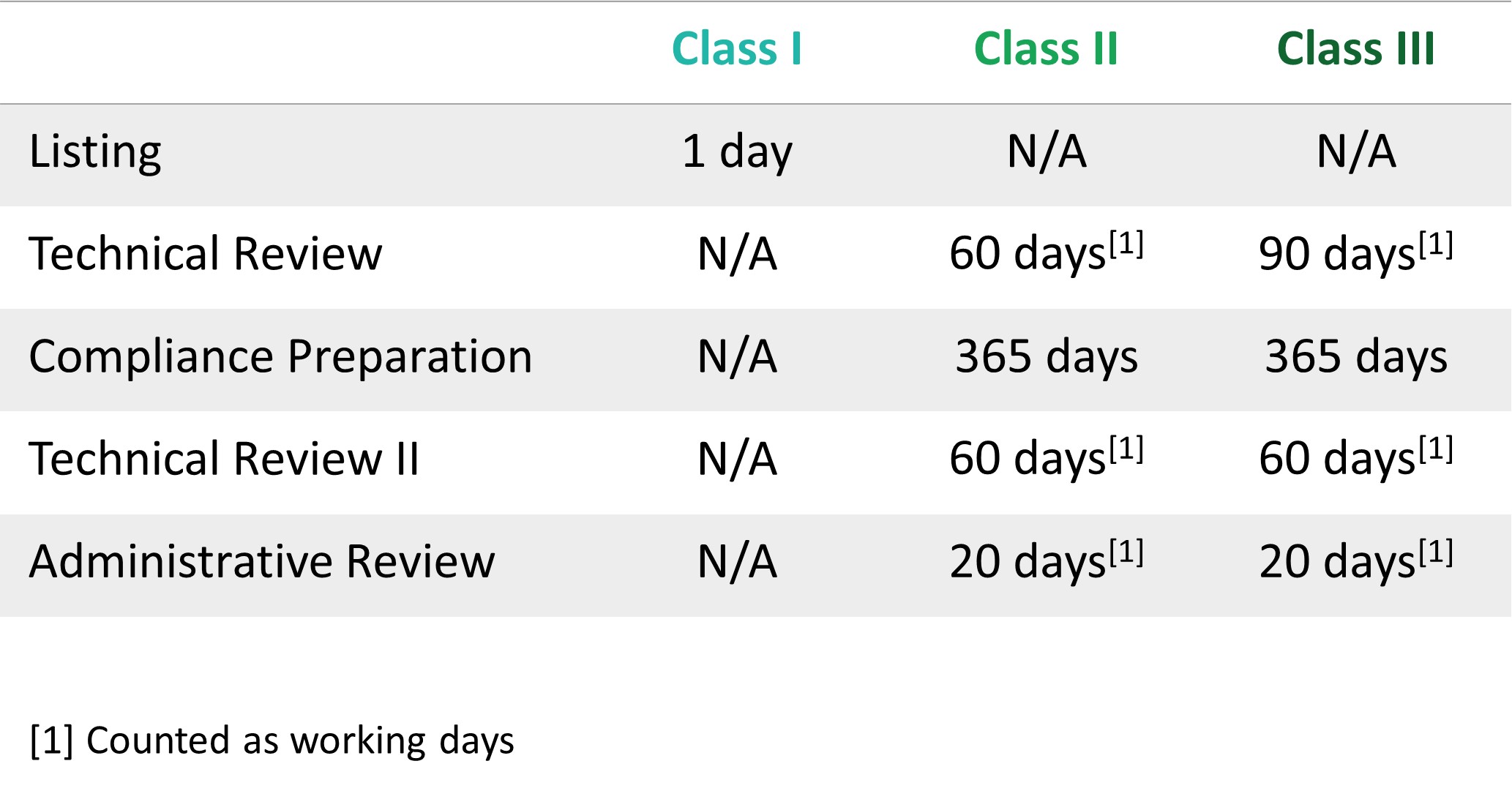
| TECHNICAL REQUIREMENTS | ||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
Note: Kindly see the No.47 Order issued in 2021 for more details. |
|
|
Validity
|
| Types of Certificates: Listing, Certificate Listing: Permanent Certificate: 5 years |
Many manufacturers have the question “Should I conduct clinical trial?” Actually NMPA offers two routes to prevent your product from clinical trial as below:
List of devices exempted from clinical evaluation: NMPA has updated this Notification No.33, 2023. If your product FULLY meet one item in the list, your product has opportunity to avoid submitting clinical evaluation report, but only submit SE comparison with NMPA approved similar product.
Clinical Evaluation Report: You can compare with predicate product based on China CER regulation and provide sufficient evidence. It’s important that the evidence shall be public or authorized by the manufacturer of predicate device.
NMPA can accept the oversea clinical trial report in degree. The registrant shall comprehensively explain the difference of China GCP and the GCP that oversea clinical trial complying with.
For more information, please refer to the NMPA’s official website at NMPA Website or you may contact us for a free consultation.
我們透過Cookies蒐集您的瀏覽記錄,以了解您如何使用我們的網站,從而分析及改善您的體驗。如繼續使用我們的網站,即表示您接受我們使用 Cookies。