January 15, 2018
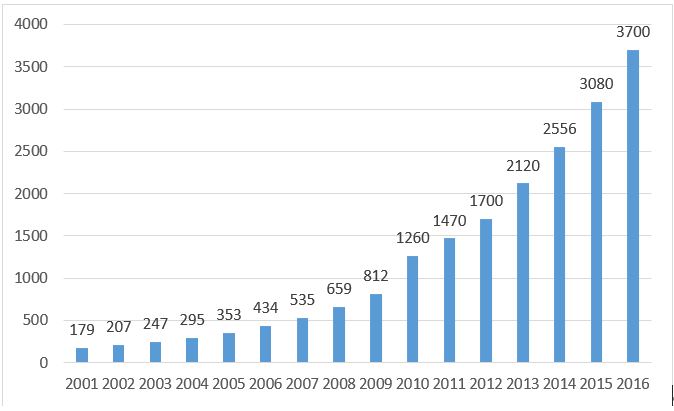
According to"2016 China Medical Device Industry Development Bluebook " released by China Medical Pharmaceutical Material Association, the medical device market size in 2015 was around 308billion RMB, while in 2016, the market size reached 370 billion RMB with an increase rate at 20.1%. Around 72.7% of the total market sales is contributed by hospital-use medical device whereas personal-use medical device is 27.3%. Another index to show how fast-growing the medical device market is by reading the domestic medical device manufacturing sector growth. In first-half of 2017, it grows 12.6% comparing to 2016 with total revenue at 142 billion RMB, according to Ministry of Industry and Information Technology of PRC.
Figure 1: The market scale of medical device (from: China Medical Pharmaceutical Material Association)
To further support how strong the demand is for the medical devices in China, there are about 990 thousand healthcare facilities by the end of July, 2016 as China National Bureau of Statistics recorded. Among them, there are 28 thousand hospitals which public and private hospitals accounts for 12870 and 15470 respectively. Comparing to 2015, there is a significant increase in private hospitals at 2115 with the total increase of hospitals 1168. As CFDA’s records, there were 14,151 manufacturing enterprises and 186,269 distributors in China at the end of 2015. By the end of 2016, there were 15,343 manufacturers and 335,725 distributors in China. Consequently, the number of medical device enterprises has grown significantly.
Table 1: The statistics of medical device enterprise (from CFDA database)
|
|
Manufacturing enterprise |
Distributor enterprise |
|||
|
FY |
Class I |
Class II |
Class III |
Total |
|
|
2007 |
3245 |
7233 |
2123 |
12601 |
160952 |
|
2008 |
3368 |
7533 |
2240 |
13141 |
157364 |
|
2009 |
3696 |
7869 |
2311 |
13876 |
155765 |
|
2010 |
4015 |
7906 |
2416 |
14337 |
165203 |
|
2011 |
4051 |
8174 |
2405 |
14630 |
168596 |
|
2012 |
4095 |
8247 |
2586 |
14928 |
177788 |
|
2013 |
4218 |
8804 |
2676 |
15698 |
183809 |
|
2014 |
3966 |
9355 |
2848 |
16169 |
189833 |
|
2015 |
5080 |
9517 |
2614 |
14151 |
186269 |
|
2016 |
4979 |
8957 |
2366 |
15343 |
335725 |
Most of Medium and High End Medical Devices Are Imported
According to China customs, the scale of China's medical equipment trade was 122.65 billion RMB in the first half of 2016 with growth rate at 3.3% comparing with the same period in 2015. Within the year, the importation sales was around 58.3 billion RMB, and went up by 9.87% same period last year. The major export countries were from the United States, Germany and Japan, accounting for 60.9% of the total import value. Diagnostic equipment was greatest import product category. Eight out of ten from imported medical devices are diagnostic equipment, including X Ray tomography instrument, optical ray apparatus, color ultrasonic diagnostic equipment and other high-end equipment.
Table 2: The statistics of importation and exportation of medical device in 2016. (From: China Custom)
|
Category |
Export |
Basis |
Import |
Basis |
|
Total |
6.4324 |
-2.1 |
5.8312 |
9.9 |
|
Medical Dressing |
0.7761 |
-9.5 |
0.117 |
-7 |
|
Single Use Device |
1.0218 |
-2.4 |
0.8515 |
10.6 |
|
Diagnostic & Treatment |
2.8938 |
-3.4 |
4.2315 |
12.4 |
|
Healthy |
1.5223 |
4.7 |
0.4355 |
-8.5 |
|
Oral & Material |
0.2184 |
0.5 |
0.1885 |
15.2 |
Table 3: The statistics of Importation countries in 2016. (From: China Customs)
|
Country |
Import |
Basis |
|
|
Global |
5831.2 |
9.87 |
|
|
1 |
US |
1907.1 |
12.01 |
|
2 |
Germany |
907.4 |
4.75 |
|
3 |
Japan |
735.8 |
15.45 |
|
4 |
Mexico |
235.95 |
17.2 |
|
5 |
Ireland |
226.85 |
14.77 |
|
6 |
Switzerland |
169.65 |
-6.17 |
|
7 |
UK |
146.25 |
-8.26 |
|
8 |
Singapore |
143.65 |
6.55 |
|
9 |
French |
143 |
26.18 |
|
10 |
Korea |
113.75 |
7.83 |
What Regulations shall Medical Device Manufacturers know before export to China?
Importation of medical devices in China requires careful study of the following regulations: 1) The Law of The People’s Republic of China on Import and Export Commodity Inspection, (2) The Regulations for the Implementation of the Law of the People's Republic of China on Import and Export Commodity Inspection, and (3) The Management of the Inspection and Supervision for the Imported Medical Devices.
Besides the government regulations to follow, each provincial custom has their own guideline to supervise the inspection on imported medical devices.
What are the requirements and procedures for Medical Device Importation?
First, medical device must obtain the registration certificate from CFDA before import take place. A qualified importer shall have Business License and Importer’s License with scope mentioned in medical device. For custom clearance, it is required to provide and declare the following documents to the authority.
1. Business License and Organization Code Certificate,
2. The ten - bit code and the letter of entrustment for the record of foreign trade operators and the declaration for customs declaration
3. HS CODE;
4. The registration certificate and the registration form of medical devices issued by the CFDA.
5. Trade contracts, packing list and invoice
6. The photos, labels, specifications of equipment, the IFU in foreign language and in Chinese, and the end-use of the medical equipment;
7. Other requirements from custom
In addition, according to the 26th directive released by the General Administration of Customs in 2004, "Automatic import license management measures", some devices need to have automatic import licenses. To find out whether your device is required to apply for such license, please see the catalogues of automatic import license management.
What is the registration procedure in China?
Medical device registration process is highly regulated by CFDA and it’s a laborious process. CFDA categorize the medical devices into three classes. Class I medical devices are not required for registration certificate to import but must notify to CFDA prior. It is compulsory to obtain registration certificates for Class II and III medical devices.
For class II and III medical devices, the foreign manufacturer shall appoint a local company as the legal agent to process the product registration in China. With the product technical specification provided by foreign manufacturer, the local entity shall send the product for testing to any of the 53 Test Centers accredited by CFDA. Whether a clinical trial is required or not would be depended on each product. Upon test report and other statistic data required by Authority are obtained, the local entity may file the registration application.
There are two phases in product registration, technical review and administrative review. Center for Medical Device Evaluation (CMDE) is responsible for the former while CFDA is latter. Only the medical devices passed by both reviews shall be granted with Product Registration Certificate.
Qualtech Service
Qualtech established the Beijing office in 2008, and received the national GCP in 2016 and 2017. We offer one-stop service, including clinical trial services, registration of foreign medical device, and license holding services. We intend to provide you not only professional and efficient medical device regulatory consultation but also a strategic solution by serving as your local authorized representative. We are ready to be your best partner to enter China market and grow your business. Are you ready?
Reference:
- The statics of CFDA in 2015
- The statics of CFDA in 2016
- The FY2013 annual report of Ministry of Healthy
- Automatic import license management measures
- China National Bureau of Statistics
- The annual statistics of the Ministry of industry and information technology
-
The guidance of medical device industry from Hong Kong Trade Development Council