Founded in 2000, Qualtech Consulting Corporation has been providing medical device manufacturers with high quality services in Regulatory Consulting, Product Registration, Clinical Trials (CRO), Local Authorized Representation, and Post-Market Activity.
Why choose QUALTECH?
Increase Your Global Outreach
Qualtech will help expanding your business worldwide. With over 20 years of experience in the medical device market and its international network, Qualtech offers an enjoyable journey for our clients with a great variety of services.
Receive Professional Assistance
Our team consists professionals from multiple fields. The team constantly receives necessary updates on new products and market regulations, providing a one-stop solution.
Enjoy high-quality service
Qualtech is a customer-oriented company with highest standards. We offer services without delay and address every case by assigning a dedicated account manager backed by our great tech center.
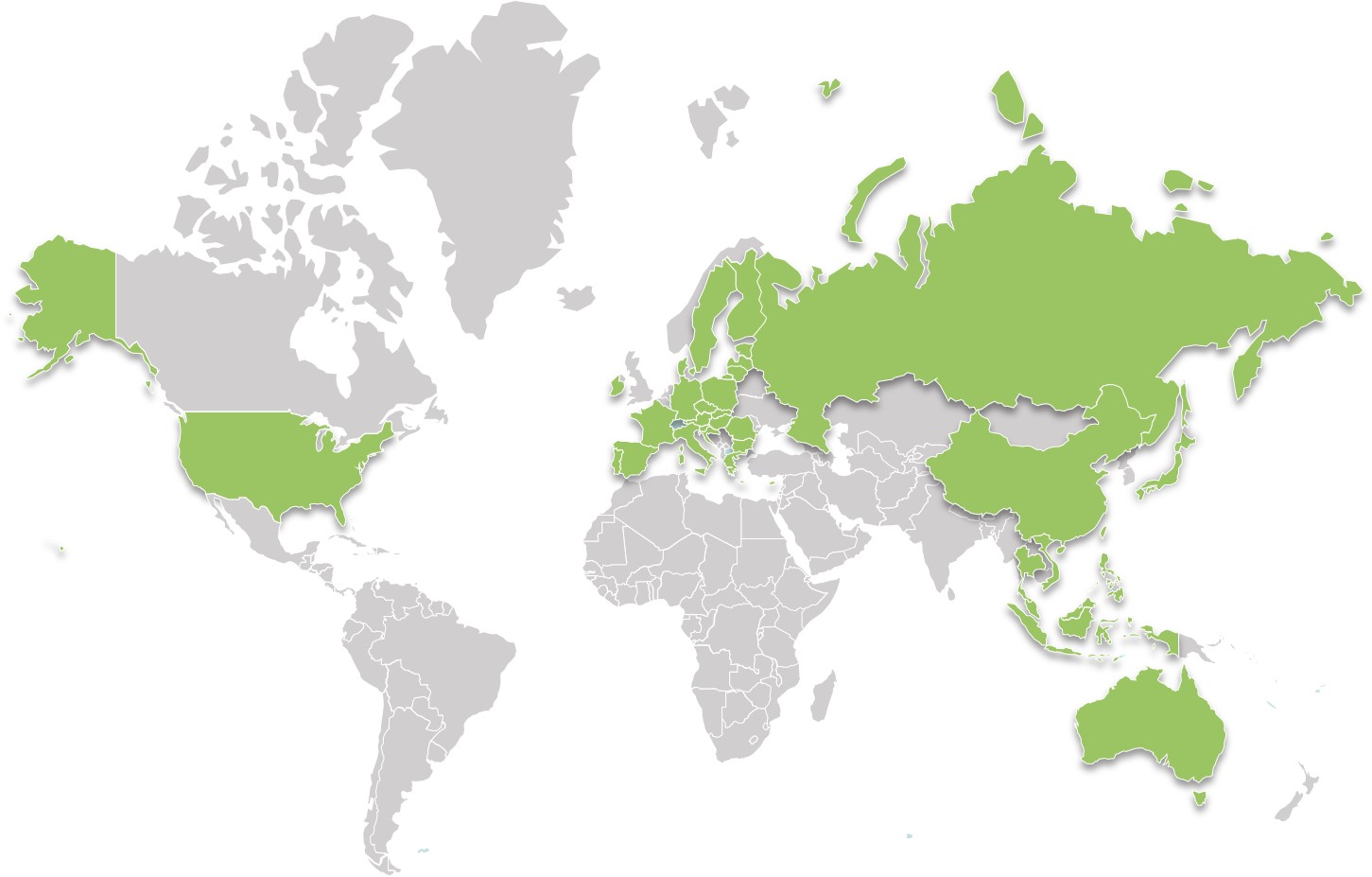
QUALTECH now has a local representation in 12 markets worldwide. Our teams in China, United State, Japan, Singapore, Taiwan, Malaysia, Philippines, Hong Kong, Indonesia, and Vietnam will help you achieve your goals with real-time services.
Qualtech Memorabilia
Establish Head Office in Taipei
2000Establish Beijing Office; The 1st Consultant Obtaining TFDA GCP Approval
2008Establish Singapore Office
2012Establish HongKong Office
2013Establish Malaysia Office
2014TFDA GCP Approval
2015Establish Philippines Office; China FDA GCP Approval
2016Establish Japan Office and Indonesia Office; China FDA GCP Approval
2017Establish Vietnam Office; China FDA GCP Approval
2018Establish New Office, Taiwan GDP Enterprise
Establish Thailand Office and United States Office Become the 1st batch of Taiwan GDP verified enterprise
2019We collect your browsing history through cookies to understand how you use our website to analyze and improve your experience. By continuing to use our website, you accept our use of cookies.
