Introduction:
Local health authorities in Asia tend to open regulatory fast tracks for medical devices that are urgently needed in the country. As the world is facing the COVID-19 pandemic, special expedite routes have been opened for related devices and equipment in order to more efficiently counter the spread of the disease. Allowing certain products to be exempted from the regular registration routes and, in some cases, even being eligible to be imported directly. Below Qualtech will therefore take a closer look at the emergency registration channels implemented in China, Singapore, Malaysia, and Indonesia and will inform you on what kind of products qualify.
Besides these novel fast tracks, also the regular fast tracks for urgently needed devices along with the eased importation policies will be introduced.
● 1) China:
China's NMPA has approved 72 anti-epidemic medical devices within the past 2 months using its regulatory fast track, including medical facial masks, medical protective clothing, medical isolation beds, respirators, and infrared thermometers, in accordance with the medical device emergency approval procedure. These products are quickly marketed through the so-called “Notice of Emergency Medical Device Approval Process” (hereafter named “Notice”) implemented by NMPA in 2009 to assist in epidemic control or domestic public health events.
The purpose of this “Notice” is to effectively prevent, and to timely control and eliminate the harm of public health emergencies and to ensure that the medical equipment needed for public health emergencies is available on the market as soon as possible. All classification of products, whether domestic or imported, can be marketed through this fast track. Manufacturers who want to apply for this approval route need to provide the product descriptions, instructions and test reports issued by China's official testing institute. Qualtech of course knows that the validity duration of the acquired license represents a key point for all manufacturers. Domestic Chinese manufacturers shall take the local FDA’s notice as their reference concerning this matter. The validity period of emergency licenses for foreign manufacturers; however, varies for each Chinese province. Generally, NMPA has not yet declared a specific validity period for the foreign product licenses once the emergency situation has been resolved. Therefore, we advise our readers to look for NMPA’s further notice or to closely follow Qualtech’s news during the next weeks and months for updates. Nevertheless, at least for protection clothes a clear validity period has been declared by NMPA, allowing manufacturers of related products to maintain the emergency license for one year.
Table 1: The turn-around time for each classification in the emergency process.
|
Classification |
Technical Review |
Admin. Review |
|
Class I |
5 days |
|
|
Class II |
5 days |
3 days |
|
Class III |
10 days |
3 days |
In this COVID-19 virus epidemic, NMPA has also released related product guidelines. Besides assisting manufacturers in developing related products, the guidelines ensure that the local FDA agencies are consistent in their standards for reviewing emergency products. The published guidelines include "Review of New Coronavirus Nucleic Acid Detection Reagent Registration Technology", "Review of New Coronavirus Antigen / Antibody Detection Reagent Registration Technology" and "Review of Pneumonia CT Assisted Triage and Evaluation Software". During the most tensed period of the outbreak in China, NMPA had also allowed a number of foreign products to be imported into China without registration.
At this critical moment, there are a number of innovative products that stand out – among them is GE Healthcare’s second generation of its intelligent imaging analysis platform technology, LK 2.0. The product claims to combine AI technology with image processing and image recognition methods to provide intelligent analysis of CT imaging of the new pneumonia software platform. However; so far, China’s NMPA has not yet listed AI-based lung CT diagnostic platforms, and this LK2.0 is currently only used for scientific research.
Fig1 : LK2.0 Platform (From GE Healthcare Press)
China further adopted relevant import liberalization measures in February to encourage more overseas-listed products to support the COVID-19 epidemic. The branch FDAs in major metropolitan areas of the country, such as Beijing, Tianjin, and Chongqing, have allowed emergency importation of foreign products before they have registered. However, these channels were closed again in March. In the future, manufacturers who had imported products under this emergency rule are asked to either complete registration within a certain period of time, or otherwise will need to re-export unsold products.
References:
●The Approval Status of the Anti-COVID-19 Device by NMPA
●The Approved List of New Coronavirus Nucleic Acid Detection Reagent
● 2) Singapore:
2.1. Introduction of the Registration Channels for Urgently Needed Medical Devices:
With the sudden increase in demand, also Singapore’s HSA has announced flexible document requirements for the import of hand sanitizers, masks, thermometers and protective gear. HSA reiterated that hand sanitizers do not need HSA approval while masks, thermometers and protective gears require notification only if it exceeds certain limits and/or depending on intended use (personal, commercial, workplace use, donation, etc.).
This announcement follows the sudden surge of demand for these medical devices in the country due to the COVID-19 outbreak. Hand sanitizers fall under the topical antiseptics’ definition of the HSA. On a separate announcement last November 25, 2019, topical antiseptics are not subject to approval and licensing by HSA for their importation, manufacturing and sales in Singapore.
Starting from Jan 31, 2020, HSA has further stopped requiring importer’s license and facilitated the importation of protective gears, which includes surgical masks, particulate respirators e.g. N95 masks, thermometers for measuring human body temperature and any protective gear for medical professionals e.g. isolation gowns and gloves. However, notification may be nevertheless required, depending on the quantity of the device and its intended use.
Regarding devices intended for personal use by an individual or a family member/s, the limits are set as follows: Masks or respirators: 150 pcs per person; and thermometers: 2 units per person. Above these numbers, HSA must be notified through the online HSA Personal Import Form.
For commercial and other purposes (such as for workplace or donations), the HSA Exemption Order Notification Form must be submitted on-line. Importers, however, may be required to submit the sales and/or distribution records. The mentioned online forms and templates can be found directly on the HSA website.
2.2. Provisional Authorization for COVID-19 Diagnostic Tests:
Given the urgent need for timely detection of COVID-19 infections, the HSA has implemented a provisional authorization process for tests intended for the detection/diagnosis of COVID-19. This provisional authorization is based on a review process that takes the design aspects of the test as well as the supporting validation data into consideration. HSA requires the submission of periodic reports including safety and performance data post authorization, as to guarantee the continued performance of these tests. In case any safety or performance issues are being detected, Singapore’s HSA will demand follow up actions from the manufacturer in question.
COVID-19 Diagnostic tests having received HSA’s provisional authorization are permitted to be delivered to private hospitals, healthcare institutions, medical clinics and clinical laboratories in the country. This fast track has assisted greatly in expanding the number of diagnostic tests available in Singapore at the moment. However, if a manufacturer intends to supply their tests to Singapore on a long-term basis beyond the current emergency situation, the tests must still be registered with HSA.
2.3. Market Presence of Innovative Devices:
In order to efficiently counter the further spread of COVID-19, innovative devices are also being developed in Singapore, one of the most technologically advanced countries globally. For this purpose, Singapore’s National HealthTech Agency (Integrated Health Information Systems - IHiS) has cooperated with startup KroniKare. Jointly, they have launched a tool involving AI-powered temperature monitors, through which automate temperature screenings can be conducted remotely.
The iThermo solution detects people with fever and informs the respective personnel immediately, while preventing the person from entering public spaces, such as governmental buildings or hospitals. This technology represents a faster and safer alternative to manual forehead thermometers, as it scans people’s temperature while they are walking by. The device has been in use in Singapore’s St. Andrew’s Community Hospital as well as the IHiS’ headquarter since the middle of February and will likely see further applications in the upcoming weeks.
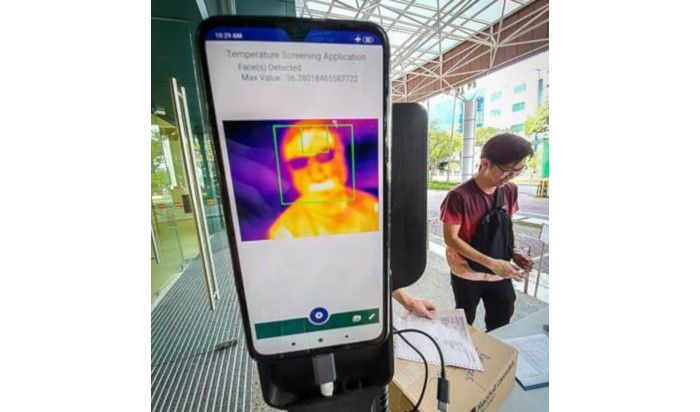
Fig2: KroniKare’s iThermo (Picture from MobiHealthNews)
References:
- HSA Announcement from Feb 14, 2020: Import of Hand Sanitisers, Masks, Thermometers and Protective Gear
- GovInsider: How Singapore built an AI temperature tool in two weeks
- HSA Announcement from March 24, 2020: HSA Expedites Approval of COVID-19 Diagnostic Tests in Singapore via Provisional Authorisation
- MobiHealthNews: AI-powered temperature screening solution being trialed in Singapore
● 3) Malaysia:
3.1. Introduction of the Registration Channels for Urgently Needed Medical Devices:
In light of the Covid-19 outbreak, MDA has allowed the following medical devices to apply for a Notification of Exemption from Registration of Medical Devices (Special Access route). Upon approval, they can be supplied unregistered, directly to healthcare institutions, in Malaysia. However, they are prohibited from being advertised in any form:
- Any brand of face masks;
- Wondfo SARS-CoV-2 antibody test (by Guangzhou Wondfo Biotech);
- Any brand of personal preventive equipment (PPE) for Covid-19 frontliners.
Under the Medical Device (Exemption) Order 2016, medical devices for special access are exempt from
