|
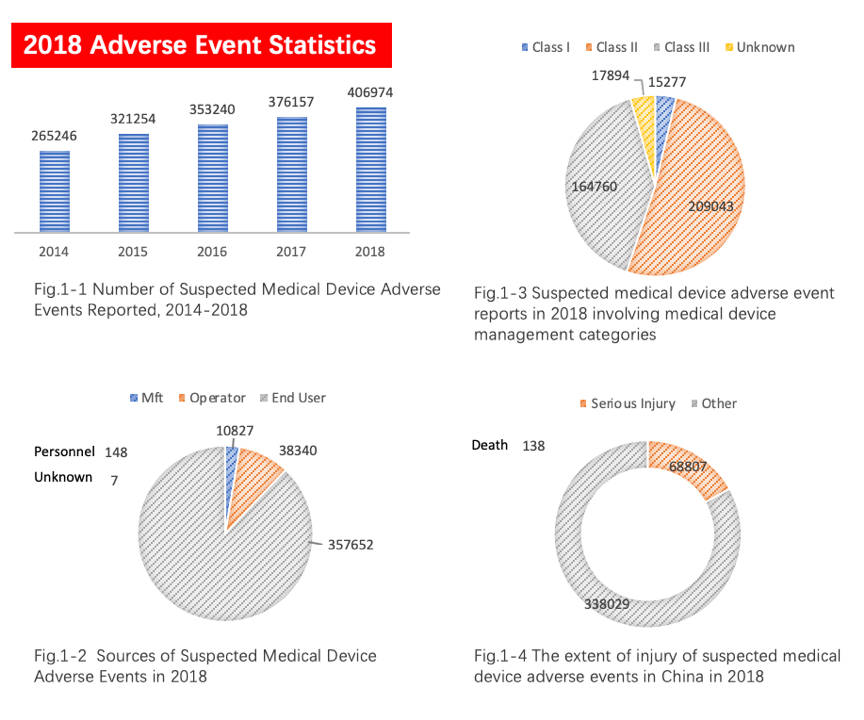
December 17, 2019 Recently on November 13, NMPA had issued a notice on the suspension and rectification of a domestic manufacturing factory,, instructing the manufacturer to stop production until rectification is completed and approved by NMPA. On the other hand, nearly 30 manufacturers (domestic and foreign) initiated voluntary recalls of their products in November. Similar news has been cropping up in recent year, and it’s foreseeable that NMPA will keep strengthening the surveillancein the future. China's post-market surveillance system is composed of two sub-systems -"adverse event monitoring system" and "post-market inspection system", which is participated by the government, manufacturers, distributors, China local agents and end users. The China government is gradually improving the post-market surveillance system and intends to cover the whole of China. According to the summary report of adverse event monitoring statistics in 2018, which was released by the China NMPA, there were over 400,000 reports of suspicious medical device adverse event with an increase of 8.19% compared to year 2017, as shown in Figures 1-1 to 1-4.
The number and quality of adverse event reports are closely related to China's surveillance system. The above statistical results reflect the China government's efforts in increasing stakeholders and users’ awareness of adverse events in medical devices. The orderly conduct of inspection on distributed products plays a role in the "defense system" in the post-market surveillance system of medical devices.
Fig.2 Conditions for initiating the flight inspection Measures for Inspections of Medical Devicesdescribesthe inspections conducted by NMPA to confirm the authenticity, reliability, and compliance of medical devices during their overseas development and production process. NMPA selects at random the inspection objects, and the proportion of random inspections of imported products has also increased year by year. In order to allow manufacturers and auditors to have a consensus on inspections procedure, NMPA has also issued the related guidance for public perusal.
Overseas Inspections on Manufacturing Site Overseas Measures for Inspections are applicable to medicines and medical devices that have been listed or proposed to list in China. NMPA is responsible for the overseas inspections and is in charge of medicines and medical devices. The Center for Food and Drug Inspection of NMPA (hereafter named as “Center”) is responsible for the specific organization and implementation of these overseas inspections. Center will send the "Notice of Overseas Inspection" to the targeted manufacturer or its China agent. The manufacturer shall submit a letter of authorization and “Basic Information Sheet for Overseas Inspection Products” to the Center within 20 working days from the date of receipt. In addition to that, the manufacturer shall also submit the Manufacturing Master File within 40 working days. Before that, the manufacturer must designate a China agent and issue a power of attorney in accordance with relevant requirements. The China agent would assist on communication between the NMPA and the manufacturer, monitoring of adverse events of the medical device, and product retrospective recalls.
Inspection on Product Sold on Market NMPA has been carrying out post-market product quality inspection since 2011, and has released a draft of the "Management Measures on Quality Inspection of Medical Devices" in this year August, which is expected to be officially implemented in 2020. NMPA is responsible for the organization and implementation of the product inspection on medical devices and the province CFDA branch carries out the supervisions and random inspection within its administrative areas. They typically focus on products of high risk, heavy amount of use, and posing possible quality and transportation problems (shown in Figure 2). NMPA will sample these products from clinical users or distributors and verifies the product quality, according to the products’ technical requirements. The China license holders are required to cooperate with the NMPA to carry out these product inspection, and shall prepare the product registration certificate, the product technical requirements, the relevant records of production, operation and use, and the entrusted production agreement of medical devices and other materials. The designated agent of the overseas manufacturer shall cooperate in the inspection of imported medical devices. The manufacturer and the respondent shall, upon being informed of the non-compliance of the product, perform the following obligations: (1) To carry out product recall and issue product recall information; (2) To carry out self-inspection and rectify relevant problems; (3) To take necessary risk control measures according to the investigation and evaluation.
References:
Tags: China, Adverse Event, Product inspection, Post-market Surveillance |
我們透過Cookies蒐集您的瀏覽記錄,以了解您如何使用我們的網站,從而分析及改善您的體驗。如繼續使用我們的網站,即表示您接受我們使用 Cookies。