The medical device industry in Philippine is an excellent investment prospect. The market growth continues to bring opportunities to foreign manufacturers of high-value, low-volume, cutting-edge, high-quality products. Qualtech is your ideal partner for your foray into the medical device market in Philippine with a practical and seamless registration process.

Qualtech in Philippines
Possess a valid Importer LTO from FDA Philippines
Medical device enterprises will require a valid LTO issued by the Food and Drug Administration Philippines to be able to apply for product authorizations (registration or notification) and import regulated products.
Specific in High Risk Implants
Qualtech Philippines team has assisted over 100 implantable products to obtain PFDA licenses, including cosmetic filler, surgery suture, bone grafts, and so on.

Medical Device Registration
Our in-house experts can offer the outstanding professional support in polishing the tailored ASEAN Common Submission Dossier Template (CSDT) and liaising with PFDA personnel to have your devices registered in the Philippines.

Authorized Representation
Qualtech can hold a medical device registration license on behalf of overseas manufacturers targeting to market medical products in the Philippines as an in-country authorized representative (AR) or market authorization holder (MAH). This is in accordance with the law for a local establishment to hold a license.
UPDATED REGULATIONS
PFDA implemented FDA Circular No. 2020-001 (Initial Implementation of Administrative Order No. 2018-0002 “Guidelines Governing the Issuance of an Authorization for a Medical Device Based on the ASEAN Harmonized Technical Requirements”) in March 2020.
In the subsequent years, FDA Circular No. 2021-002 has been issued, and revised with its latest update FDA Circular 2021-002-C.
Salient Points of the Current Regulations:
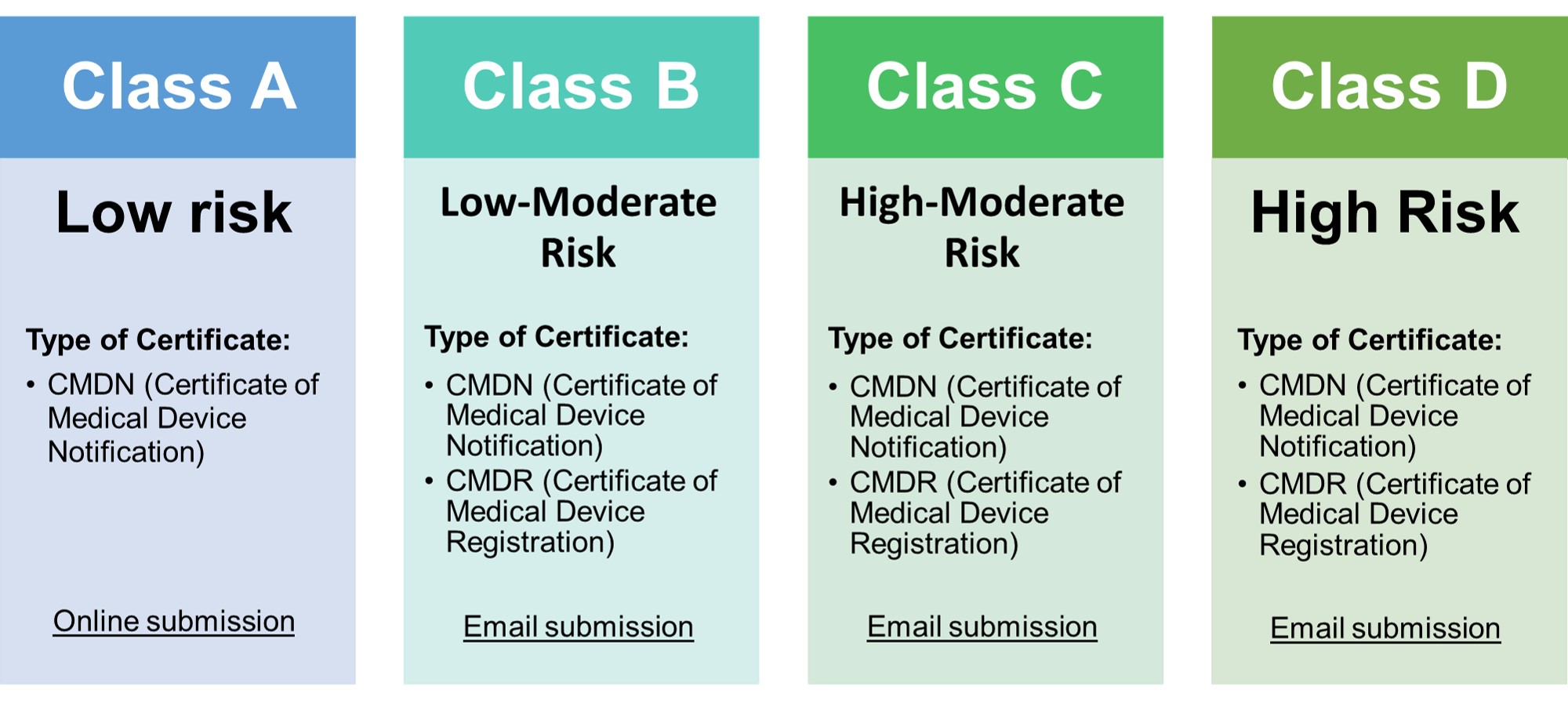
- • Classification of medical devices now follows the ASEAN MDD
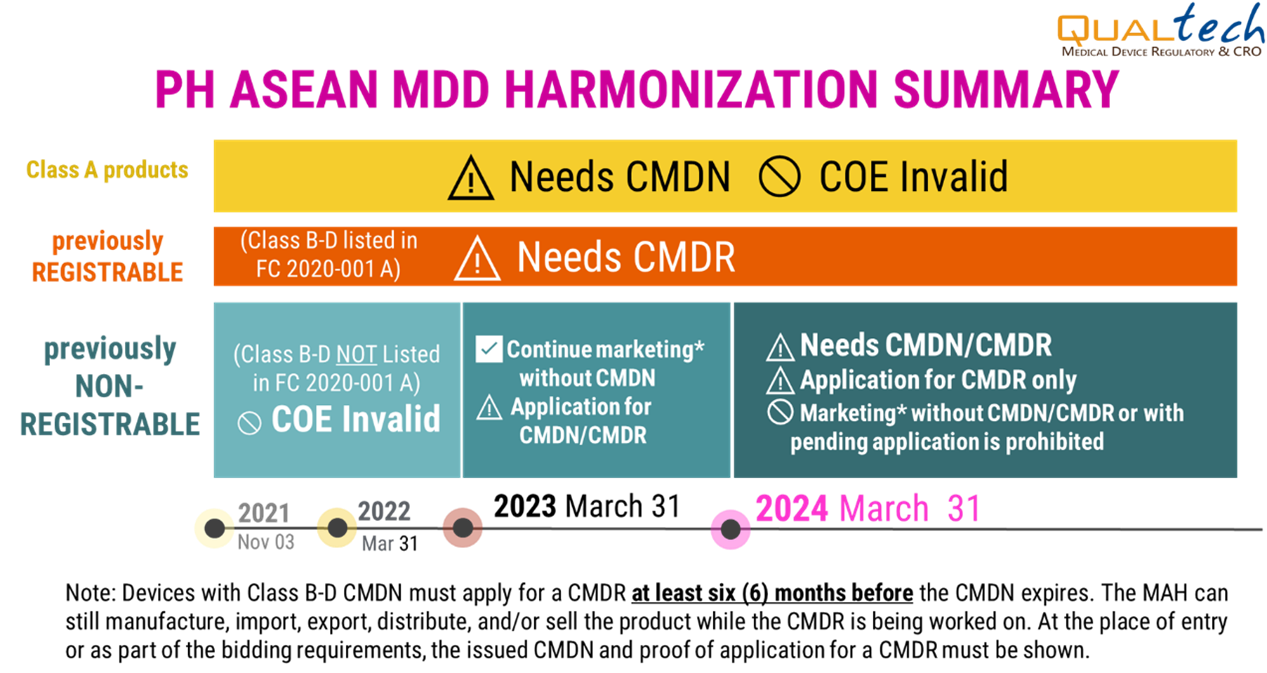
- • Class A medical devices need to have a certificate of medical device notification (CMDN).
- • Class B, C, D devices that were considered registrable in the FDA MC No. 2014-005 need to have a certificate of medical device registration (CMDR).
- • Previously considered non-registrable devices now falling under Class B, C, and D may still undergo notification (CMDN) application until the 31st of March 2024. Afterwards, registration (CMDR) will be a requirement for all such devices.
- • Medical devices which are NOT listed in FDA Circular 2020-001-A should be classified according to the ASEAN MDD
- • For devices to be used solely for research, clinical investigation, exhibit, personal use, sample product for analysis/testing, or donated brand new medical devices) and is not intended for sale shall require a listing (CMDL).
- • COE is NO LONGER a valid document; all devices must undergo notification, registration, or listing.
-
Timeline:
- Updated as of July 2023
- Classification
|
|
|
|
|
|
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Note: Kindly see the Annexes B – E of DOH AO 2018 – 0002 for more details.
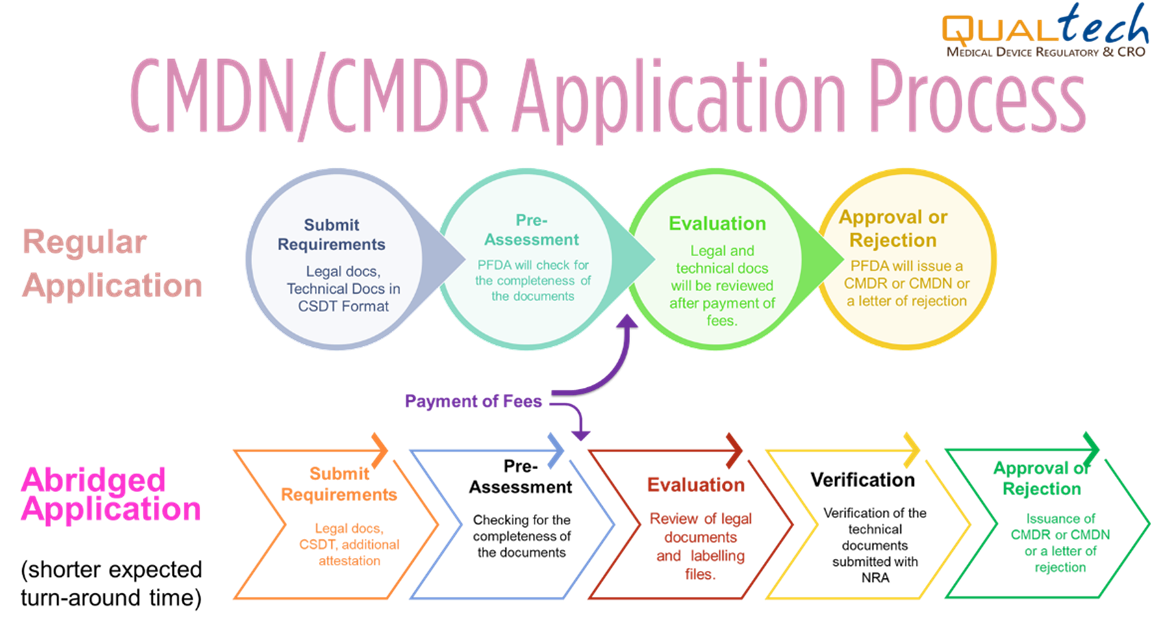
- Workflow
FDA Target Reviewing Evaluation Time
After successful internal evaluation of the submission dossier, our in – house experts will then submit it to the FDA and will be subjected to thorough evaluation. Below is a list of the evaluation times for different types of transactions involving product registration/notification.
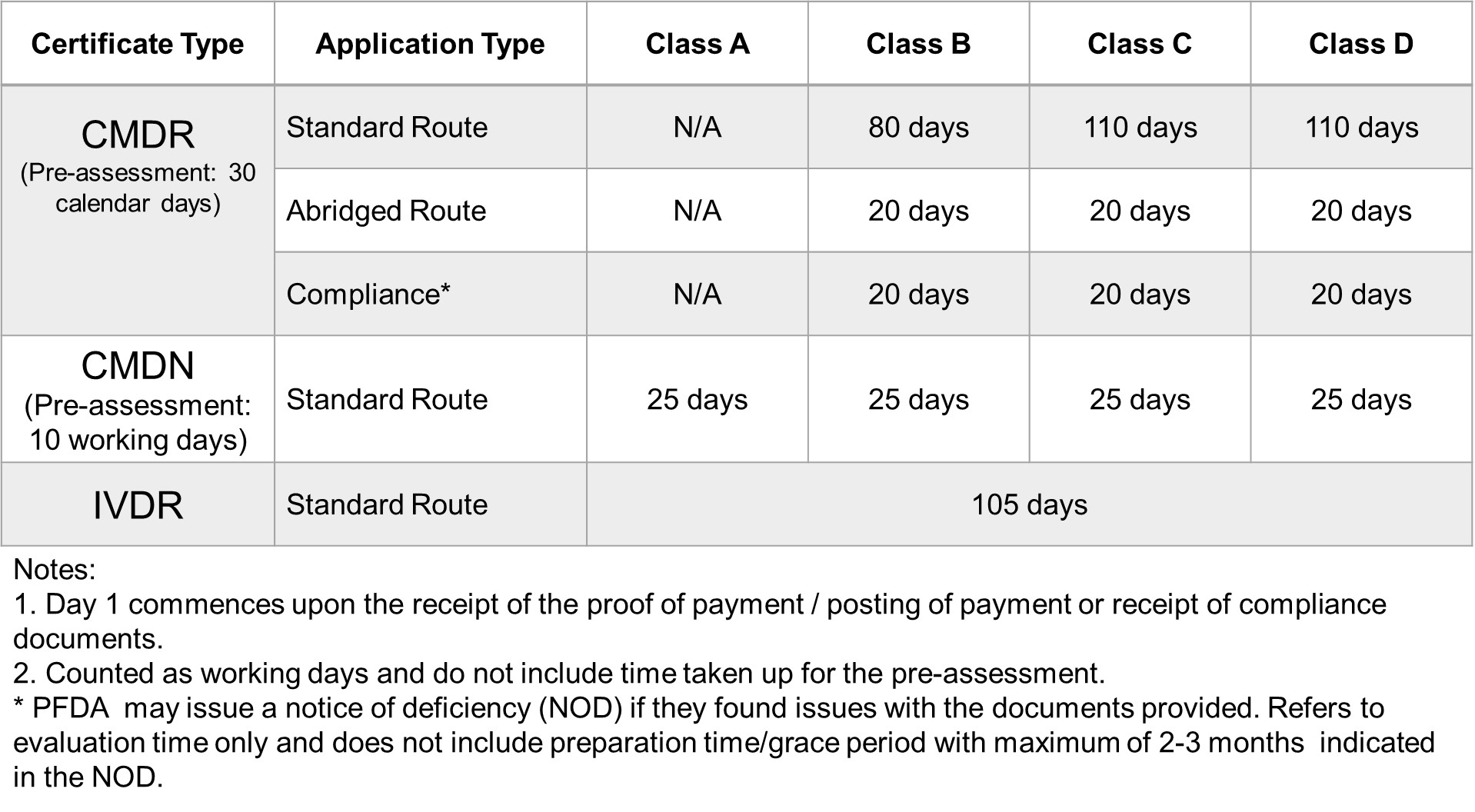
Abridged Processing
For medical devices that are covered under Administrative Order No. 2018-002, all applications for registration of medical devices approved by the National Regulatory Agency (NRA) of any ASEAN member country under the AMDD-CSDT can apply for Abridged Processing.
The technical requirements to be submitted to the PFDA shall be the latest and the same as those submitted to the reference agency where the Certificate of Product Registration (CPR) was issued, and if the product applied to the FDA is the same medical device with no changes after prior approval, the device is eligible for Abridged Processing.
|
|
CMDN |
CMDR |
|
Class A |
5 years |
- |
|
Class B, C, D |
2 years |
5 years |
PFDA application shall be made separately for each specific medical device. However, filing one application that will yield multiple certificates is applicable in the following conditions where the medical device.
• with accessories that are intended to be sold separately,
• owned by the same manufacturer, but manufactured in different manufacturing plants and will be both distributed at the same time in Philippines,
• system where the use of one part of the system is needed to be used together with all or any part of the system,
• with the same intended use and manufacturing process but differ in one or more raw materials,
• with the same intended use and manufacturing process but differ in the design,
• with the same raw materials but differ in types or shapes resulting in different specific intended use.
For more information, please refer to the FDA Philippines’ official website at http://www.fda.gov.ph or you may contact us for a free consultation.
• Dipping into the Healthcare Market of the Philippines - Facts, Opportunities and Imminent Changes
• PFDA Issues Guidelines for the Initial Implementation of Administrative Order 2018-0002
Wir verwenden Cookies, um Ihnen ein optimales Website-Erlebnis zu bieten und um die Zugriffe auf unserer Internetseite analysieren zu können. Klicken Sie auf "Ich stimme zu", um Cookies zu akzeptieren und direkt zur Website weiter zu navigieren.

