|
February 14, 2020
On January 23, 2020, PFDA released FDA Circular No. 2020-001 as initial implementation of Administrative Order No. 2018-0002 (Guidelines Governing the issuance of an authorization for a medical device based on ASEAN Harmonized Technical Requirements).
The list of registrable medical devices has been updated and classified according to risk level (A-D, low to high risk level). Some of the devices in the FDA Memorandum Circular No. 2014-005 and 005-A has been added, others deleted or reclassified or added with further descriptions. The higher the risk class, the stricter the documentation requirements. Specific provisions are listed in below:
- Devices with risk class B-D that are included in FDA Memorandum Circular No. 2014-005 and 005-A must be applied for a Certificate of Medical Device Registration (CMDR). Class B-D devices not included in this list and IVDs not listed in the same circular and FDA 2020-001 will be considered non-registrable.
- For those under class A, even those not included in FDA Memorandum Circular No. 2014-005 and 005-A should be applied for a Certificate of Medical Device Notification (CMDN).
- Brand new or donated devices used for research, clinical trial, exhibit, personal use devices must have Certificate of Medical Device Listing (CMDL).
- Previously issued Certificate of Exemption (COE) for medical devices for class A in the updated list shall only be valid until November 3, 2021.
- Existing Certificate of Product Registration (CPR) for class A devices shall be acknowledged as equivalent to CMDN but only until the validity of the CPR. CMDN will be issued upon renewal of CPR of such devices.
- COE will no longer be issued for non-registrable products but in case of point of entry and/or as part of bidding requirements, the License to Operate of the establishment should be provided.
- In case of devices not included in the updated list, the ASEAN Agreement on Medical Device Directive shall be applied.
- The validity of the initial approval was extended to five years with corresponding increase in application fee, from 1500 to 7500 pesos plus 1% legal research fee (LRF). The renewal interval and fee has been adjusted likewise adjusted, every five years and the cost is now 5000 pesos plus LRF. CMDL fee is the same as previous COE fee, 500 pesos + LRF.
The list of registrable in-vitro diagnostic device (IVD) is the same as in FDA Memorandum Circular No. 2014-005 but blood collection tube has been added to the list.
The effectivity of this new guideline will be 15 days after publication in a newspaper of general circulation and upon acknowledgement of receipt of copy by the Office of the National Administrative Register. No publication or acknowledgement of mentioned office has been released as of article writing.
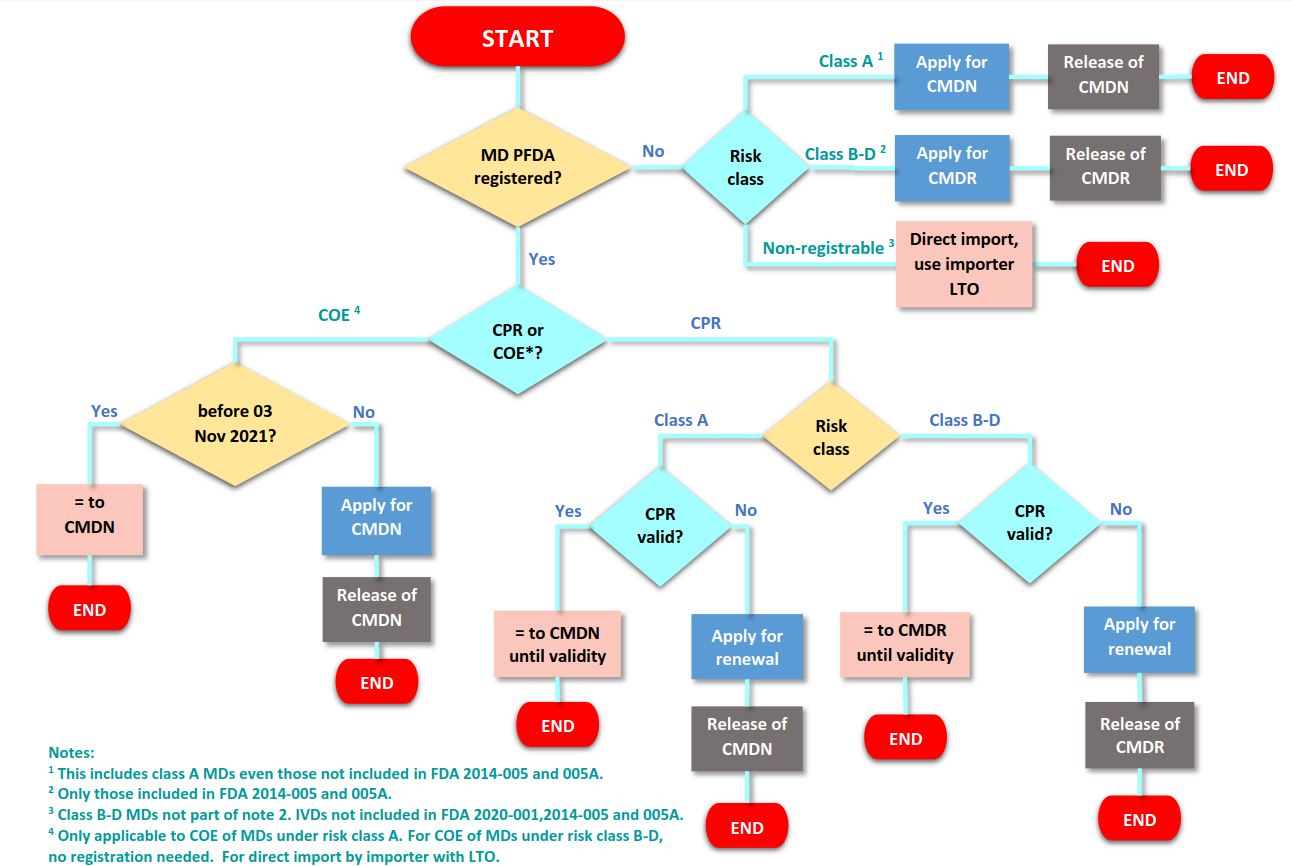
Figure 1: FDA Circular 2020-001: Initial Implementation of AO 2018-002
References:
FDA Circular No. 2020-001
|

