August 13, 2018
Revised Guidelines for Telehealth Products
June 2018 marks the implementation of revised guidelines for Telehealth medical devices set by HSA in Singapore.
The following terms were given modified definitions in the latest revision of the said guideline:
1.) Wellness device:
“A device or software which is intended by its Product Owner to be used only to enable or encourage the user of the device or software to adopt or maintain a healthy lifestyle, or for the user’s general wellbeing, but not to be used for any specific medical purposes.”
Wellness devices are not considered as medical device by HSA. This definition has implication for those devices that are not intended to be used as wellness devices but is able to perform such function/ purpose (e.g. monitoring heart rate). For these devices, manufacturers are required by HSA to include the following clarification statement on their labels:
“This device or software is intended for use only for general wellbeing purposes or to encourage or maintain a healthy lifestyle, and is not intended to be used for any medical purpose (such as the detection, diagnosis, monitoring, management or treatment of any medical condition or disease). Any health-related information provided by this device or software should not be treated as medical advice. Please consult a physician for any medical advice required”
2.) Standalone mobile applications:
“Standalone Mobile Applications refers to a software and/or mobile application that is intended to function by itself and are not intended for use to control or affect the operation of other hardware medical devices.”
Example of these devices includes applications with algorithm based calculators of specific parameters for use in clinical practice or for use in diagnosis or managing a disease or condition. Such applications are required to be designed based on formulas with established scientific evidence and clinical utility
Telehealth medical devices are subject to the following regulatory controls of HSA:
1. Product Registration
2. Dealer’s license requirements
3. Post market obligations
Product Registration: The changes in the evaluation route for different risk classes also impacts registration of telehealth devices. For instance, Class A telehealth devices are exempted from registration to HAS. Other changes in the evaluation route in the registration of other risk classes are shown in the following table:
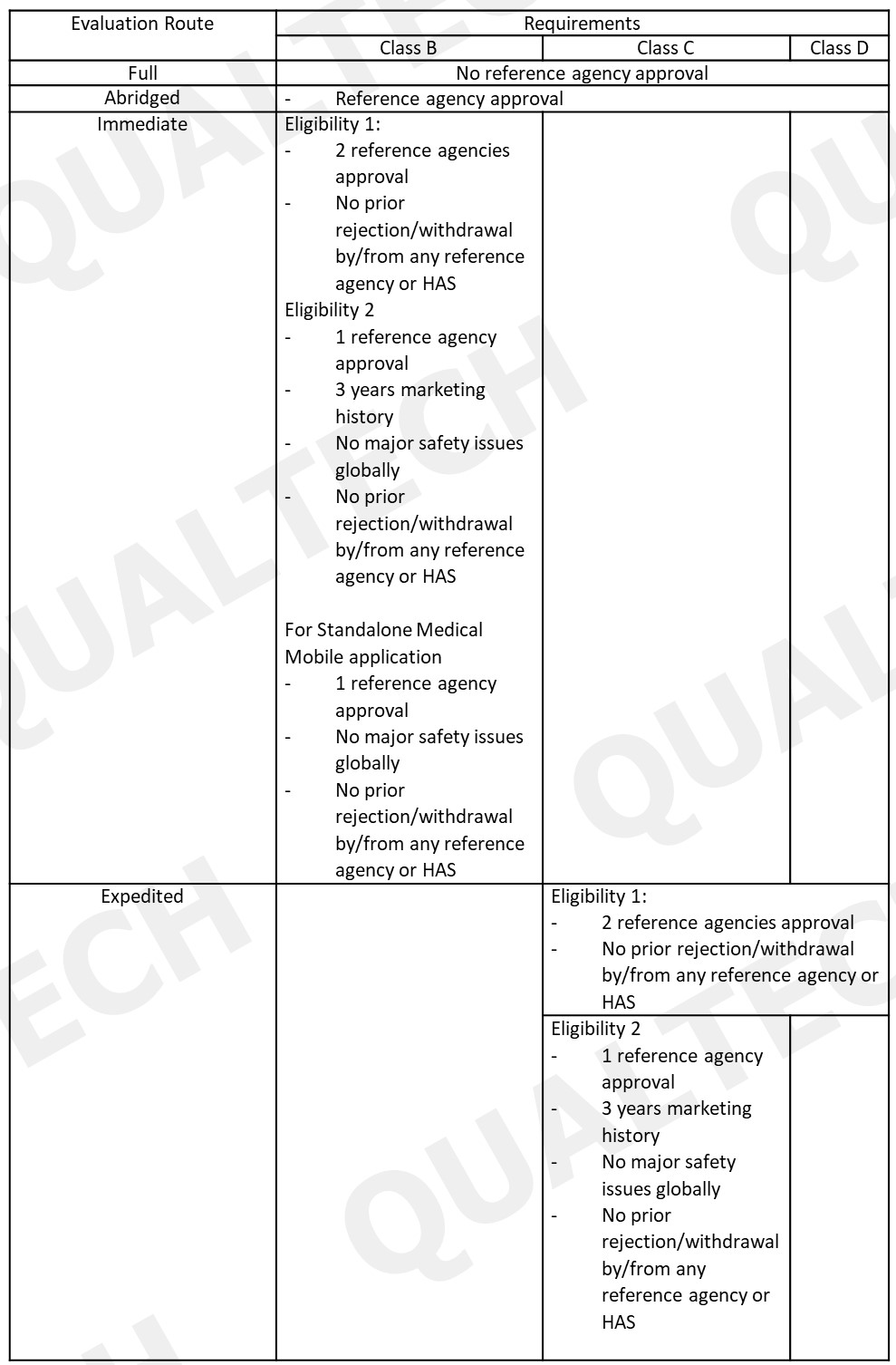
Dealer’s License Requirements: Telehealth product dealers who wants to enter the Singapore market needs to comply with GN-02: Guidance on Licensing for Manufacturers, Importers and Wholesalers of Medical Devices.
Post-market Obligations: Dealers are required to report adverse events, defects and recall to HSA and ensuring appropriate investigation, so as to ensure the continued safe use of the devices. Healthcare professionals and users of the medical devices are encourage to report to HAS regarding adverse events related to the use of a medical device or device failure related issues
New Guidelines of Devices for aesthetic purposes
HSA released a new guideline for devices used for aesthetic purposes last June 2018. Such devices that will be identified as a medical device will subjected to several regulatory controls:
$1- Product registration
$1- Dealer’s license requirements
$1- Post market obligations
Product registration: Product owners who wants to supply such devices in Singapore are required to obtain marketing clearance in Singapore.The following devices / instrument / apparatus / implement / machine or appliance that are intended for modification of appearance and anatomy or devices that are used for aesthetic purposes will be subjected to regulatory control of HSA:
The intended use of these devices set by product owner will be the factor whether it needs to be registered or not. HSA regulation is particular for medical devices with intended use on humans that includes restoring, improving or modifying physical appearance. Some examples are the following:
$1- Treatment of wrinkles
$1- Improving skin texture
$1- Skin rejuvenation
$1- Body contouring
$1- Hair removal
HSA used the global post-market surveillance and comparison of risk presented by some products to other devices in determining the list of the following high risk devices for aesthetic purposes (see Annex A).
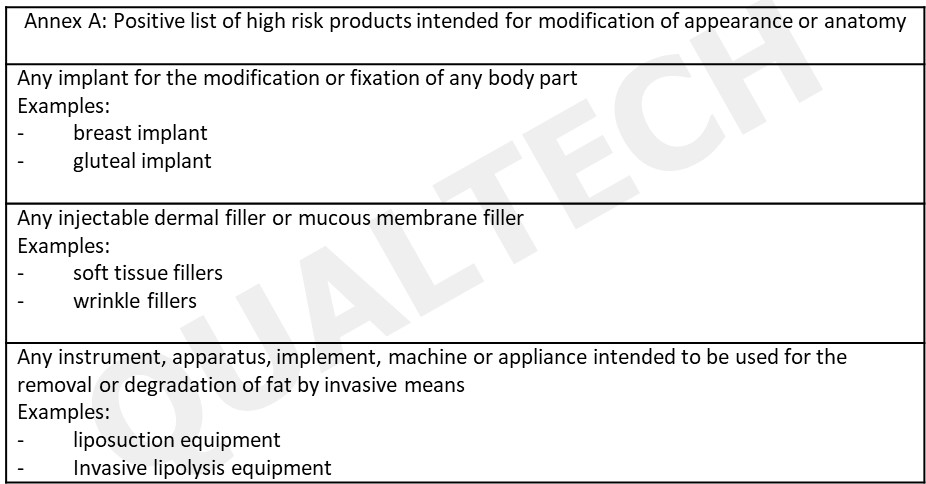
The list in Annex A will be updated in the future as more information about these type of devices are collected. Using Annex A, product owners can assess their products if these will be regulated by authority. The summarized process of determining if a device needs to be registered in HSA using the following flowchart:
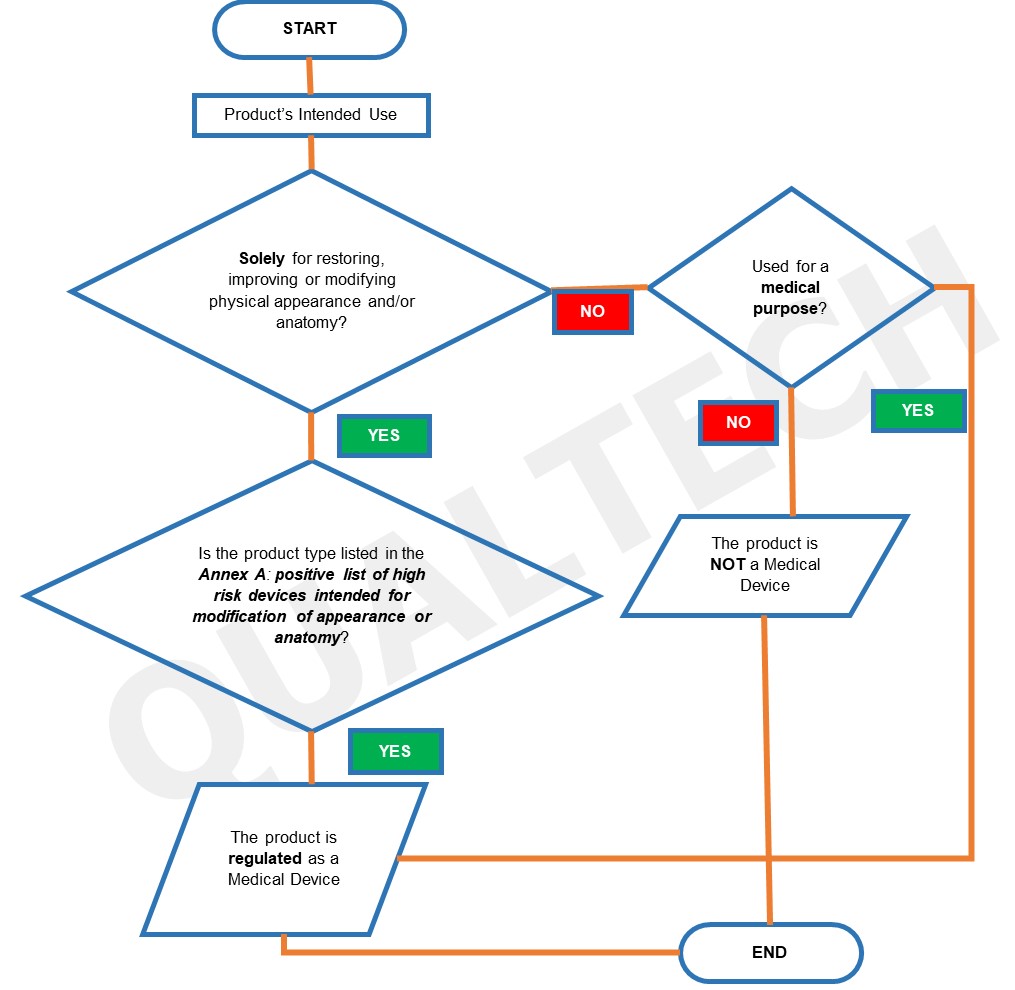
Once a device is confirmed to be a medical device based on the flowchart, the risk classification is detrermined next. For this process, owners will refer to GN-13: Guidance on the Risk Classification of General Medical Devices, depending on the nature of the device and its intended functions.
After risk classification is determined, the requirements in product registration are listed in GN-15: Guidance on Medical Device Product Registration. Part of device validation is adressing the safety concern of the device. The table below listed the concerns that are important to be mentioned in the documents. Take note that these information are similar to the ones addressed in other high risk devices. The documents listed in the table are not additional regulatory requirements.
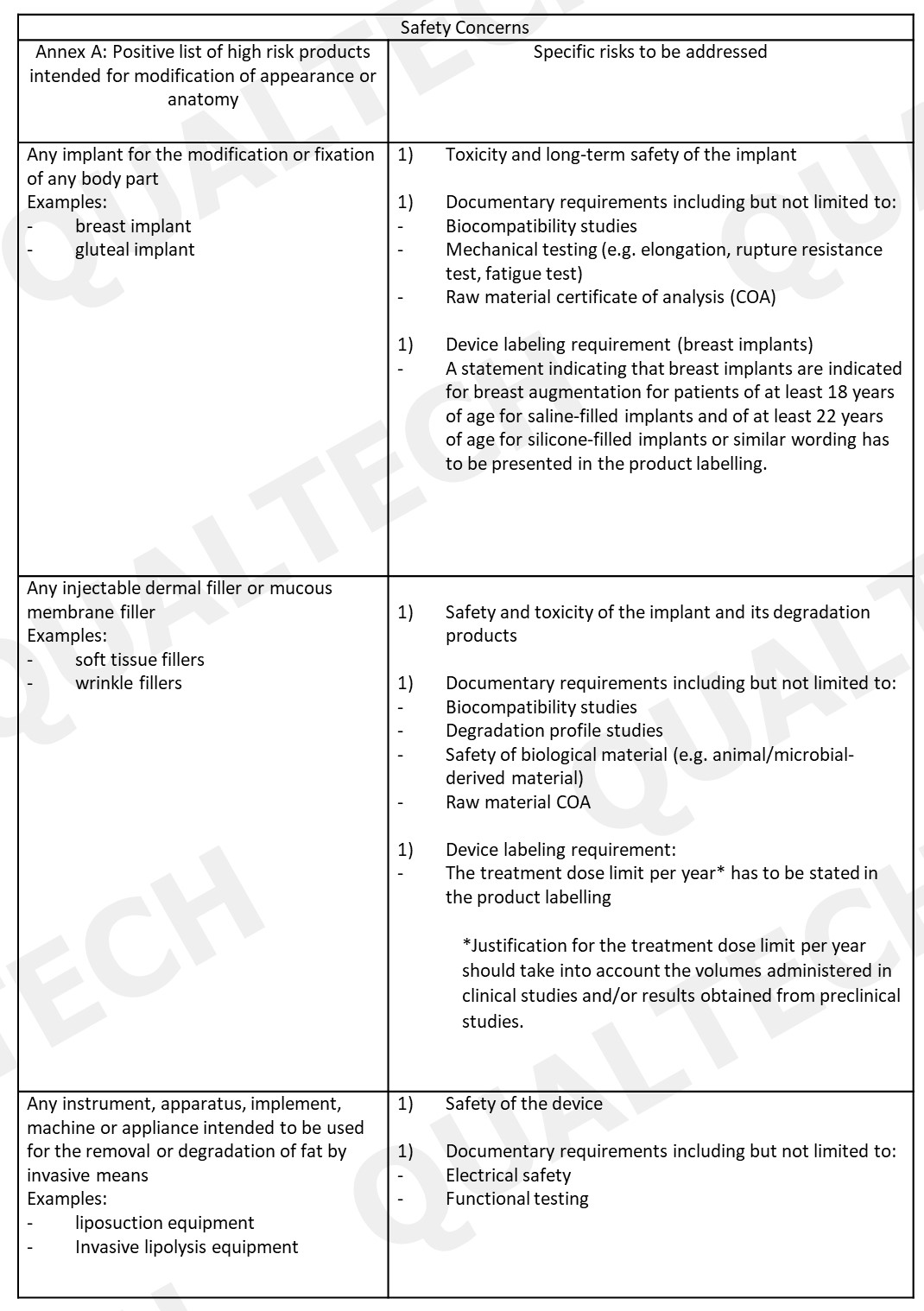
After registration, the product can undergo change or changes throughout its product life cycle. Any changes to the registered information of the device should be addressed to HAS. All the type of changes and all the requirements and steps on how to processes these are included in GN-21: Guidance on Change Notification for Registered Medical Devices.
Dealer Requirements: In order to ensure proper traceability and post-market monitoring of devices for aesthetic purposes, appropriate dealer licenses will be obtained from HAS in order to manufacture, import and/or wholesale these medical devices. Dealers will refer to GN-02: Guidance on Licensing for Manufacturers, Importers and Wholesalers of Medical Devices for requirements and steps.
Post-market Obligations: Dealers are required to report adverse events, defects and recall to HSA and ensuring appropriate investigation, so as to ensure the continued safe use of the devices. Healthcare professionals and users of the medical devices are encourage to report to HAS regarding adverse events related to the use of a medical device or device failure related issues
Conclusion: The implementation of new regulations for Telehealth products and Devices for Aesthetic purposes were implemented last June 2018. Both guidance showed what type regulatory control each device groups will have once it enter the Singapore market. Telehealth product regulation emphasized on the new eligibility criteria for shorter evaluation routes while the regulation for devices used for aesthetic purposes highlights the list of devices in Annex A that are needed to be regulated by HSA. Both of the new guidance aims to have positive impact in the market of the mentioned group of devices.
Reference:
1) Regulatory Guideline For Telehealth Products (HSA)
