Hong Kong's medical device market may be smaller compared to other famous Asian markets, but it’s an equally lucrative and expanding platform. Qualtech is your ideal partner to for your foray into the HongKong medical device market for an easy and smooth registration process.

Medical Device Registration
Our in – house experts are able to provide you with excellent professional service in preparing Submission dossiers liaising with MDD officers to get your devices registered in the HongKong.

Authorized Representation
As an in – country authorized representative (AR), Qualtech can hold a medical device registration license on behalf of foreign manufacturers looking to market medical devices in HongKong. This is in compliance with the law for a local establishment to be a license holder.
|
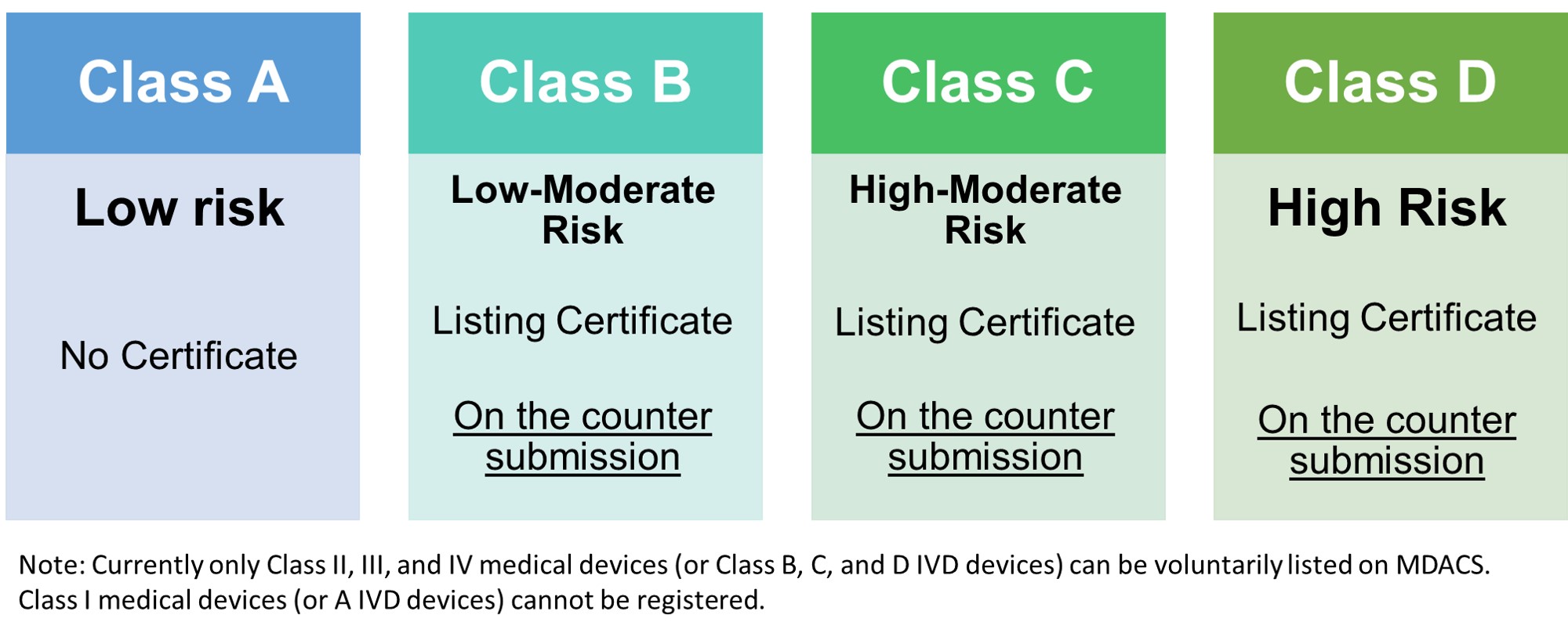
Registration with MDD |
|
|
 |
CLASS B | CLASS C | CLASS D |
|---|---|---|---|
| Essential Principles and Methods | Yes | Yes | Yes |
| Summary of Design V&V | Yes | Yes | Yes |
| Labeling and IFU | Yes | Yes | Yes |
| Risk Assessment | Yes | Yes | Yes |
| Physical Manufacturer Information | Yes | Yes | Yes |
| Clinical Evidence | Yes | Yes | Yes |
Note: Kindly see the Guidance Notes for Listing Class II/III/IV Medical Devices issued in 2022 for more details.
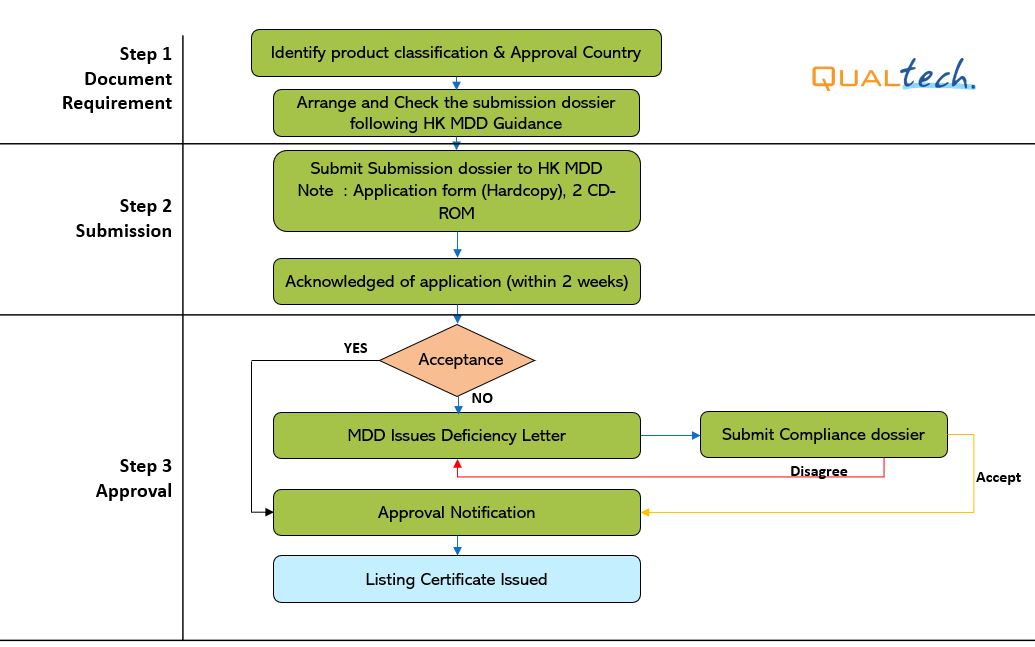
MDD Target Reviewing Evaluation Time
After successful internal evaluation of the submission dossier, our in – house experts will then submit it to the MDD and will be subjected to thorough evaluation. Below is a list of the evaluation times for different types of transactions involving product registration/notification.
|
|
|
|
- Note:
- 1. It's accounted as Working days.
- 2. The device shall get approval in US FDA, EU, Canada, Japan, Australia, China, and Korea.
- 3. The above stated working days do not include Conformity Assessment Body’s evaluation period.
-
Types of Certificates: Listing Certificate
-
Listing Certificate: 5 years
In HongKong, it's not mandatory to register the medical device before go on local market. Why does some manufacturers still decide to register their products?
Per 2023, the Department of Health has now implemented a new strategy of procurement of medical device and will give preferences to medical devices listed in the MDACS.
Sometimes the clinics or hospital prefer to use medical device approved by MDD. Therefore, medical device with MDD approval would be easier to sell in HK market.
Home-country marketing approval is not necessary, but marketing approval from one of the GHTF countries is mandatory for the device registration process.
For more information, please refer to the MDD official website or you may contact us for a free consultation.
我們透過Cookies蒐集您的瀏覽記錄,以了解您如何使用我們的網站,從而分析及改善您的體驗。如繼續使用我們的網站,即表示您接受我們使用 Cookies。