In special cases, MDA exempted medical devices from registration and allows for an Import for Re-Export (IRE) for manufacturers or importers that uses Malaysia as a logistic hub for temporary activities such as for maintenance, testing, sterilization, packaging, labelling, or temporary distribution hub where the devices are only intended to be re-exporting the devices. Applicants must be a registered company in Malaysia includes licensed establishment such as manufacturer, importer, authorised representative (AR) and forwarding agent that responsible for importation. An approval letter is issued if application met the requirements and procedures with any advertising of the IRE approval is strictly prohibited. With approval, the medical device now able to legally import the medical devices into Malaysia on temporary validity period of 12 months without entering Malaysian market.
1.0 IRE Application Process
As shown in Figure 1, before importation of devices, the applicant shall complete the Annex B IRE Application Form and submit to authority via exemption.bhai@mda.gov.my at least 21 days before the intended importation date. The MDA will process the application within approximately 14 working days. Any additional information or documents requested by MDA shall be provided within 30 days from the date of request. The applicant must include detailed information of the devices such as:
- Name of medical device
- Brand and model
- Intended use of medical device
- Manufacture information
- Total quantity to be imported
After completing the re-export, the applicant shall submit Annex C Declaration of IRE records within 30 days after date of exportation and accompanied with Customs Form No.2 (K2). The applicant needs to maintain detailed records and shall be made available upon MDA request. Non-compliance will result in cancellation of the approval letter.
2.0 IRE Subsequent Application Process
All the imported medical devices shall be exported out from Malaysia within the validity period and excess medical devices that have not been exported out shall be disposed in safely manner. Applicants may request for an extension by making subsequent applicant to MDA by completing Annex D Subsequent Application for Import for Re-export of Medical Devices to MDA via email as above at least 14 working days prior to the expiration date of current IRE approval letter. MDA typically processes complete submission within 7 working days. The extension is valid up to 6 months only from the original expiry date of IRE approval letter.
3.0 Condition of IRE Approval Letter
It is important for applicant to note that IRE approval letter comes with specific condition such as:
- Any advertising of the IRE approval is strictly prohibited.
- The approval is only valid for the specified device and facility indicated in the application.
- No further importation or exportation of the medical device in any quantity upon cancellation or expiration of IRE approval letter.
- For multiple shipments, quarterly declarations must be submitted. All records must be readily available for inspection.
- Any misuse such as diverting products into the local market may lead to severe penalties under Section 5 of Act 737, including fines of up to RM200,000, imprisonment for up to three years, or both.
This guidance offers a practical and legally compliant pathway for licensed establishment and forwarding agent to temporarily bring medical devices through Malaysia. With IRE approval, applicant can avoid registration and reduces administrative workload while remaining compliant with Malaysia regulation.
Qualtech Consulting Corporation has been a trusted partner for medical device manufacturers for 25 years. Whether you’re a local startup or an international player, we empower your devices to enhance lives. Connect with us today to unlock your medical device potential.
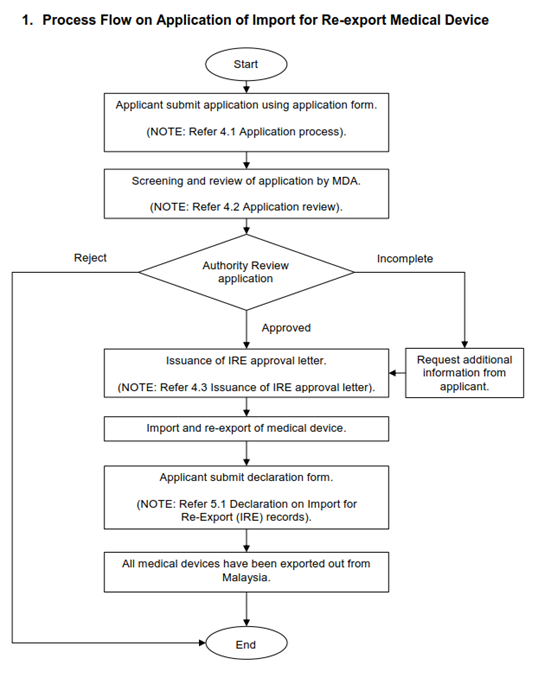
Figure 1 Process Flow on Application of Import for Re-export Medical Device: The flowchart illustrates process flow for licensed establishment or forwarding agent to apply for import for re-export medical device to obtain IRE approval letter.
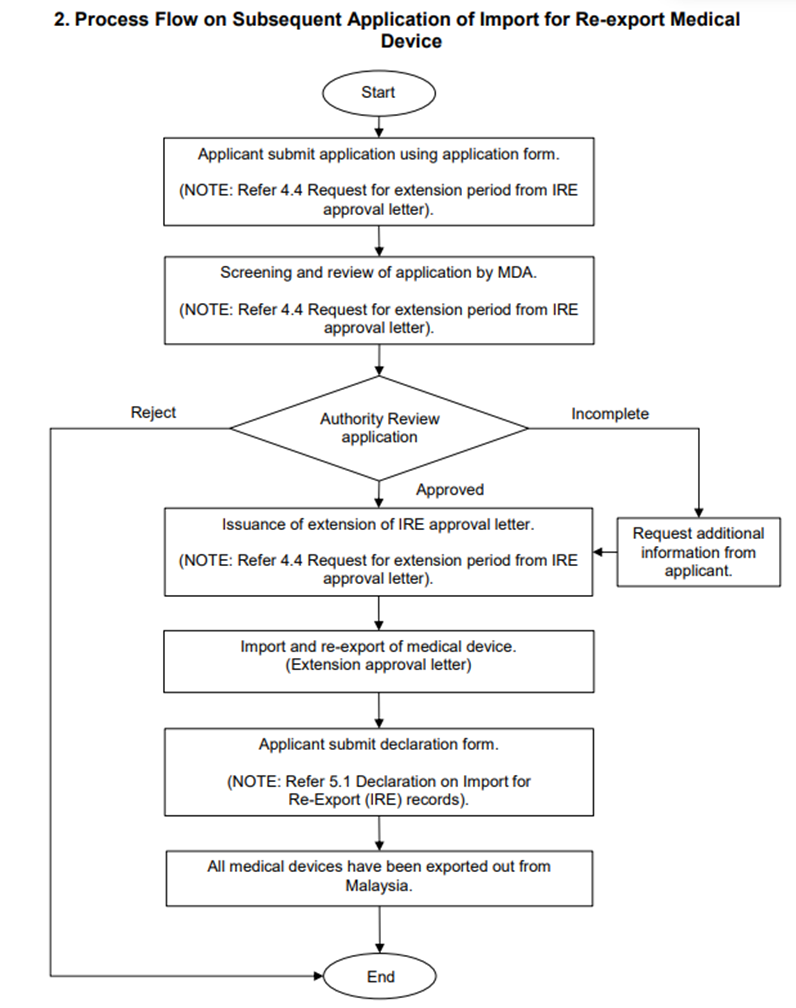
Figure 2 Process Flow on Subsequent Application of Import for Re-export Medical Device: The flowchart illustrates process flow for licensed establishment or forwarding agent to apply for extension of IRE approval letter.
References:
MDA/GD/0069 - Importation of Medical Device for Re- Export (IRE)
