December 11, 2018
Starting January 2019, Medical Device Marketing Authorization License will be issued in electronic form. This is in accordance to PMK No. 62 year of 2017 Article 22, which stated that Marketing Authorization License will not require stamp and wet signature anymore.
The implementation of the digital signature also applies to the other letter issued by MoH such as certificate of import information, CFS certificate, Certificate of Importation of Spare parts, etc. Other than, each letter and circular license is equipped with a QR Code as a security and can be verified through a mobile application.
As a common practice in previous process, before the applicant claims and retrieves the certificate, the applicant need to check the contents or the accuracy of the data printed on the certificate first. Any error information printed in certificate, must be reported to MoH before application claim the certificate. The process can be described as in the following chart:
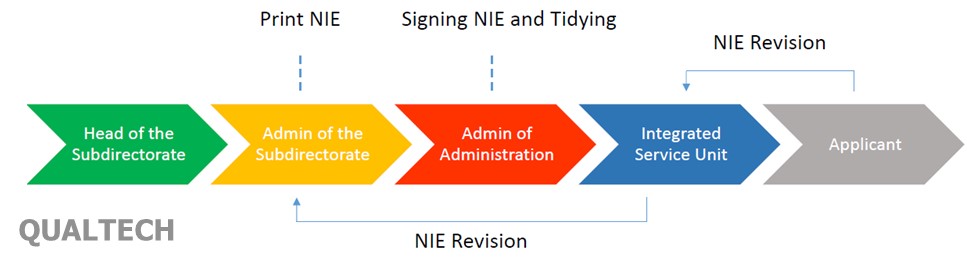
In the above process, when the applicant claim a certificate at the Integrated Service Unit counter, before the certificate is stamped by the officer, the applicant need to check the content and submit a revision immediately if there is any incorrect information written in the certificate. The applicant will be informed again when the certificate revision is complete and can be retrieved by the applicant. MoH understand that this process consume much time and work.
Therefore, in connection with the implementation of this digital signature, as well as the previous provisions, MoH would like to make the process more effective and efficient by making the changing as follows:
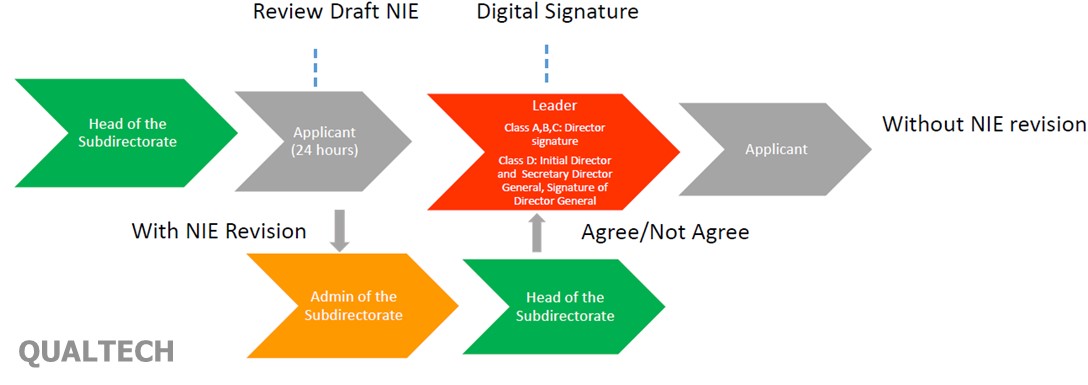
In the above process, the applicant does not need to come to the counter. Once application is being approved, MOH will notify through an email and applicants can check the contents or correctness of the data through the Digital Signature System website. Submission of revisions can only be done for 24 hours. The content which can be requested to be revised in the license are as follow:
1. Product Name
2. Type
3. Packaging
4. Factory Name
5. Country of Origin
6. Validity period
Reference:
1) Materi Sosialisasi Digital Signature dan Penentuan Kelas Secara Mandiri Perizinan Alkes dan PKRT
