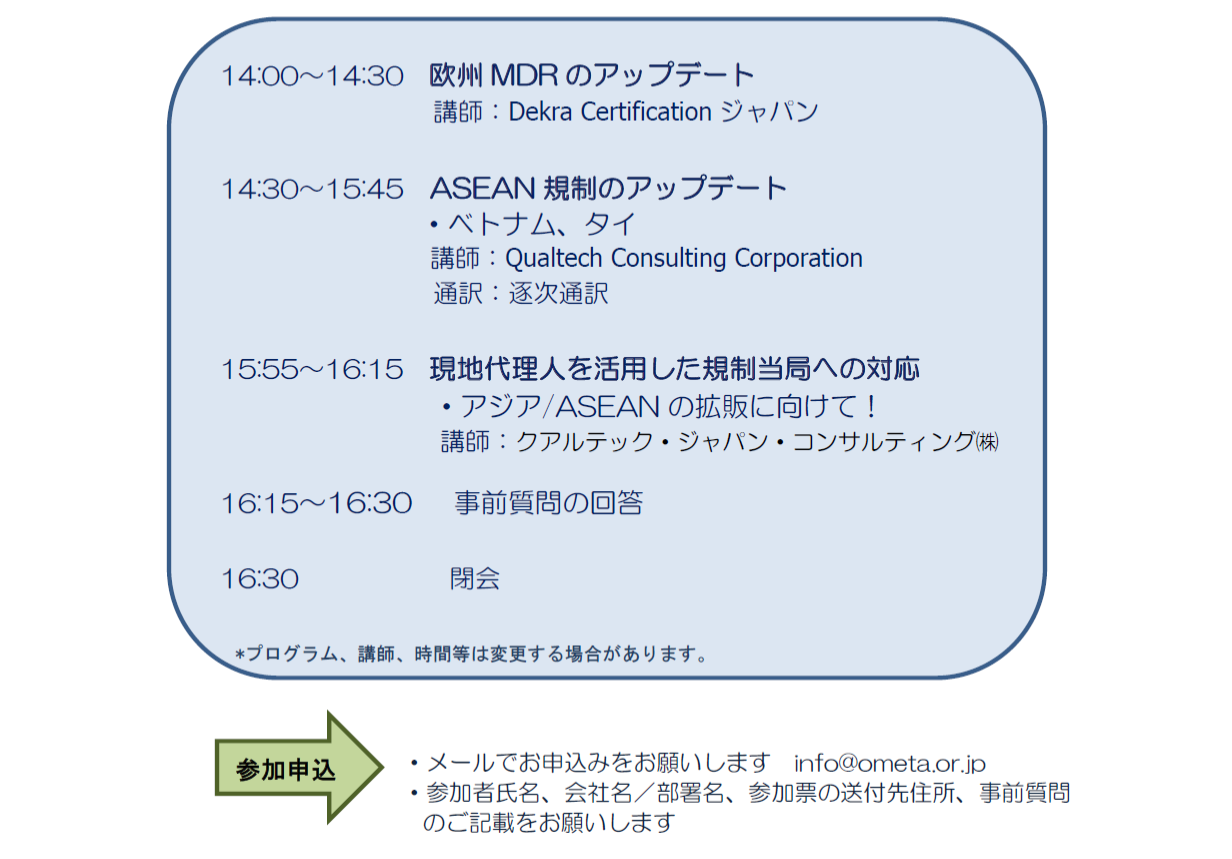
The OMETA Seminar is back in 2019, to give those interested the tools to evolve and accelerate their business. In this one-day workshop, Qualtech’s regulatory affairs specialists will walk seminar participants through the updates of the medical device regulations in Vietnam and Thailand, where Qualtech provides its registration and legal representative services. Not only will attendees receive insightful views on the ASEAN regulations, but participants will also walk away with an actionable plan to achieve their business goals in these markets.
Furthermore, Dekra Japan will provide a thorough overview of the most recent updates regarding the implementation of the MDR in Europe. Ensuring that participants’ businesses are well aware of all the upcoming changes and know how to properly prepare for the new European regulations.
【Date】
July 23rd, 2019, 2pm to 4.30pm
【Venue】
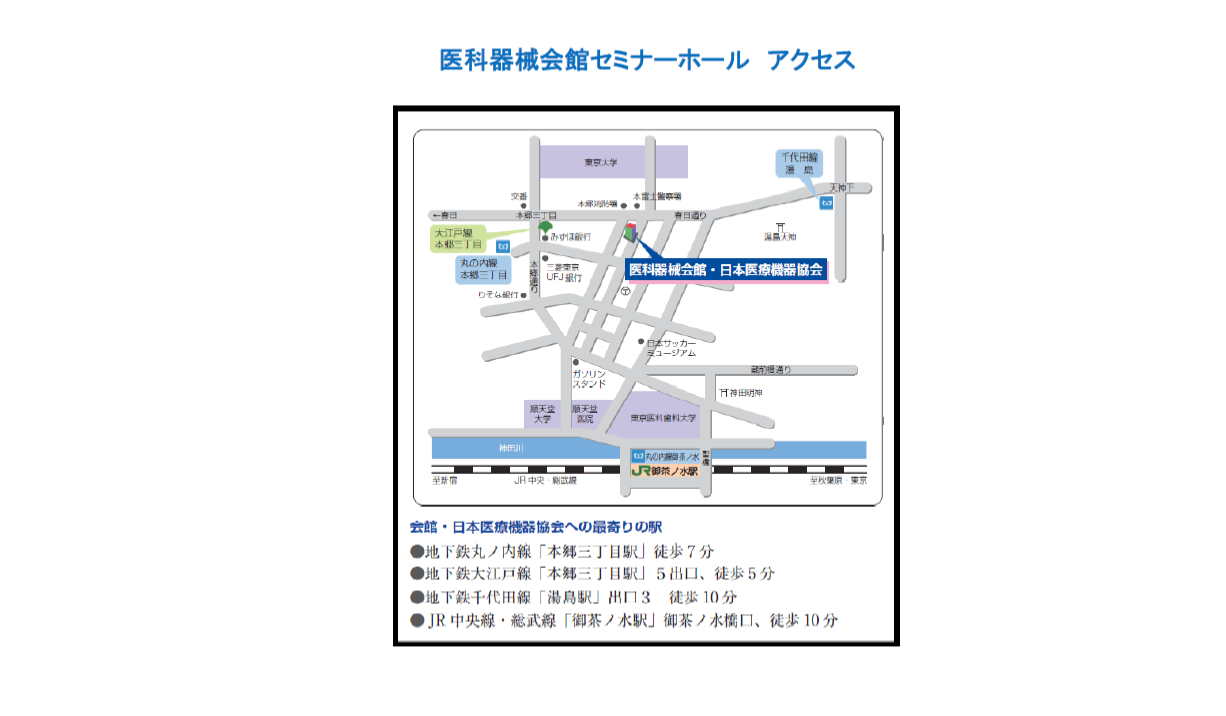
Ikakiki-Kaikan 2F (3-39-15 Hongo, Bunkyo-ku, Tokyo)
【Seminar Ticket Price】
Members: 5000 JPY
Non-members: 8000 JPY
Participants are kindly requested to send an email to OMETA at info@ometa.or.jp, with participant’s name, company name, and company address. Participants will then receive the seminar-participation slip and will also have the chance to address any questions they may have regarding the seminar topics or the event itself. Your point of contact within Qualtech Japan; on the other hand, would be Mr. Go Murayama (patty@qualtechs.com) and Ms. Aya Igarashi (aya.igarashi@qualtech.co.jp) to whom you may reach out shall you have any questions addressed to Qualtech.
Further information (written in Japanese) may be found below


References:
OMETA: Overseas Medical Technical Assistants
