In Qualtech, our goal is to serve as your reliable partner in Asia, who can provide you with professional, high-quality and efficient regulatory services within the entire region. Therefore, we are delighted to announce that the Qualtech Consulting Corporation is now operating in Bangkok, Thailand. Our services in Thailand include medical device registration, local representation and import services.
Classification
Based on the Medical Device Act 2008, medical device classification in Thailand currently still follows a policy-based instead of a risk-based approach. That is, the product classification is not yet adhering to the AMDD (ASEAN MEDICAL DEVICE DIRECTIVE), which is expected to be in force by year 2020. Therefore, medical devices are; as of now, classified as follows: (1) Notified Medical Devices, (2) Licensed Medical Devices and (3) General Medical Devices. Thailand is however gradually approaching the adaptation of a risk-based classification approach, although this has not yet officially been announced by the ThaiFDA (Thailand Food and Drug Administration).
Submission Process
The registration of Notified and Licensed Medical Devices is initially made by submitting a hard-copy dossier submission over-the-counter (OTC). Important to note is that it is compulsory to prepare the dossier according to the harmonized CSDT guidelines (ASEAN COMMON SUBMISSION DOSSIER TEMPLATE). On the other hand, the registration of General Medical Devices is done via an online submission (referred to as E-Submission), followed by a hard-copy dossier submission (OTC).
[Thailand medical device Market Overview]
a. Overview of the Thailand Health Sector
The Thai Government has effectuated its universal health insurance system in 2002 by issuing the National Health Security Act. At present, an impressive number of up to 99.95% of the population is being covered by the national health insurance system (approximately 66.05 million people). This number has increased from the previous 92.47% in 2002, with the Thai population being able to access medical and public health services through health insurance of 3 main fund programs, including:
1)The Universal Coverage Scheme: UCS
2)Social Security Fund (The Social Security Scheme: SSS)
3)The Civil Servant Medical Benefit Scheme: CSMBS
Currently, there are a total of 38,512 health facilities in Thailand, with public sectors comprising 33.9% and private sectors making up 64.4% of the total. Hospitals in major cities in Thailand’s North Eastern region are however often overloaded – with bed occupancy rate reaching 100%. This may be attributed to the unequal distribution and general shortage of health care professionals, which ultimately led to the progressive growth of private hospitals in a span of 4 years – from 321 in 2012 to 347 in 2016.
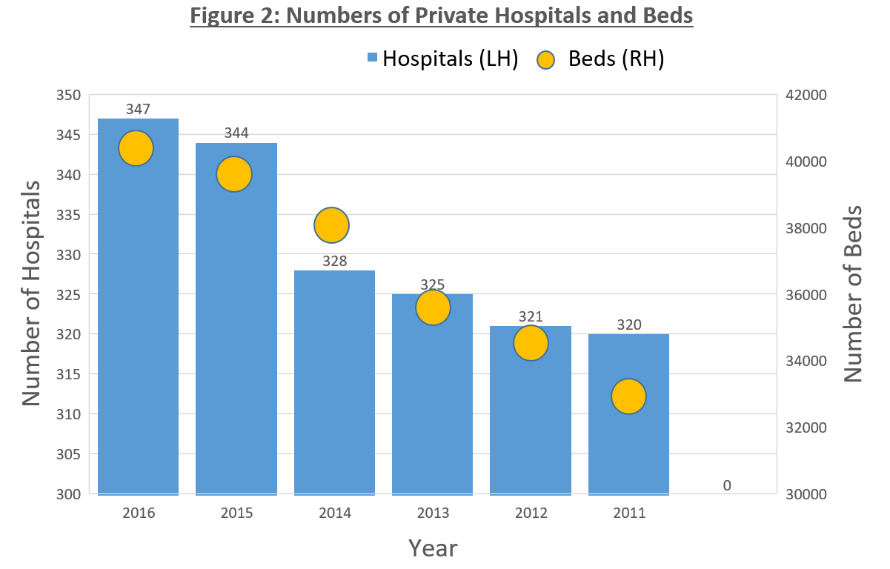
(Source: MOPH, Krungsri Research)
b. Thailand’s Medical Equipment Market
(Thailand shows potential in preparing the country to become a major Asian medical hub)
Thailand’s medical device market continues to succeed due to several key factors. The constant growth of medical tourism, the rise in the standard of living among Thai citizens, along with the government’s continued efforts to develop Thailand into a leading medical hub, all play a major role.
Thailand’s healthcare expenditure is growing annually by 6%, accounting for 892 billion THB in 2016 and is expected to reach around 12 trillion THB by 2020.
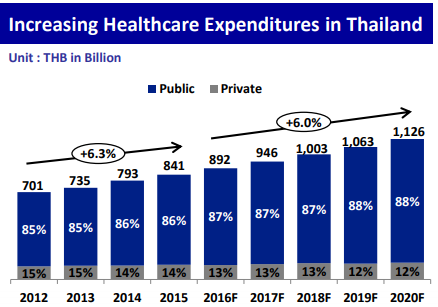
(Source: EIC SCB, World Health Organization, BMI)
Thailand has over 50,573 well-trained physicians, modern facilities and a world-class yet affordable healthcare. Costs for health services are however 50-75% lower than equal services in the US. In 2015, the rise of medical tourism has generated over $3 billion USD revenue to the country. This represented a 15% growth for the Thailand economy with an estimated 3 million medical tourists annually.
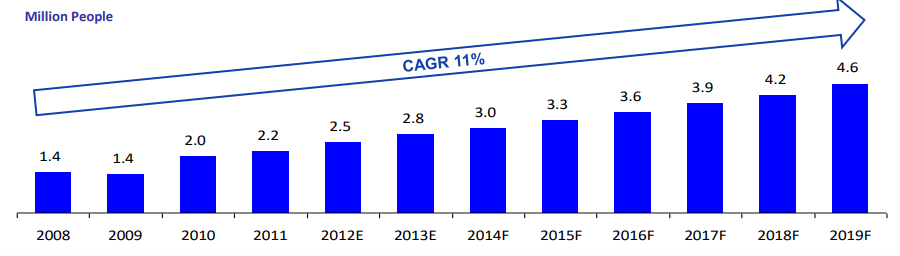
(Source: Economic Intelligence Center)
Over the span of five years (2012-2016), the value of medical devices distributed among domestic and export markets (with values split by approximately 30:70), has grown by an annual rate of 8% and 3.1%, respectively. In 2016, the total value of the Thai medical device market was THB 41.65 bn, and up by 4.7% YoY (year-over-year).
For the time period between 2018-2020, the market value of medical devices distributed domestically is projected to increase by 8.7% per year.
c. Distributor
Data from ThaiFDA reveals that there are over 2,000 registered importers of medical devices. Moreover, there are over 10,000 operators active within the sector of medical device distribution - including both wholesale and retail operations. The majority of Thailand’s medical device distributors are located in Bangkok Metropolitan Region and Chiang Mai Province.
There are 3 main distribution channels:
- Direct distribution to healthcare providers includes public- and private-sector hospitals and clinics. Procurement of medical devices by public-sector hospitals should be carried out in accordance with government procedures (commonly known as e-bidding).
- Distribution to intermediary companies, representatives or to shops. While this also includes distribution to general shops as a way of reaching target customers in the country.
- Distribution via export markets: Most goods distributed in this way are single-use devices bound for main markets in the United States, Japan, and Germany.
The only possible procurement route for public hospitals is via the e-bidding process. Since foreign companies cannot submit a tender on their own, it is beneficial to have an experienced and skilled local distribution partner with solid relationships to hospitals and to the Ministry of Public Health. Your distribution partner therefore represents a core for the market success of your device in Thailand. Apart from Thailand registration and local representation services, Qualtech therefore also helps in connecting you with an experienced and qualified distributor in the Thai market.
Conclusion
As Thailand continues to show high potential in preparing the country to become a major Asian medical hub, we may help you seize the opportunity to enter Thailand’s market by applying for an import license, managing your importation, as well as assisting you to connect with local distributors – ultimately, leading you to a smooth journey towards the expansion of your products into the Thai market.
References:
MOPH, Krungsri Research
EIC SCB, World Health Organization, BMI & Economic Intelligence Center
Medical Device Industry Research 2018-2020
