This article will cover two recent versions of GN-21: Revision 4.5 and Revision 4.6. Majority of the changes discussed here was covered in Revision 4.5.
HSA noticed that industry stakeholders often have a confusion with the terms used across regulatory guidance documents GN-15, GN-17, GN -18 and GN-21, along with the terminologies used to refer to the types of documents needed to satisfy each requirement. These typical questions received by HSA have prompted the revision of GN-21.
GN-21’s latest revision involves the following scope:
- Main Updates to GN-21 Annex 1 Change Notification
- Editorial Changes
- Clarification on Change Notification (CN) document requirements
Main updates to CN Requirements and Editorial Changes:
GN-21 has applied standardized terms and examples used across GN-15, GN-17, GN-18 and GN-21 to reduce confusion among stakeholders.
Examples:
- Standardized terms: design verification and validation documents. Clinical evidence
- Standardized examples: Accepted documents under “Proof of Quality Systems Management Certificates for Manufacturing / sterilization sites” are:
o ISO 13485 certificate
o Conformity to US FDA Quality Systems Regulations
o Conformity to Japan MHLW Ordinance 169
Another type of editorial change is a further elaboration on examples of the type of documents needed for certain requirements. In addition to editorial changes, the documentary requirements for the following CN applications has also been updated:
1.) Change in Manufacturing Facility, Process and Quality Management System (QMS)
2.) Changes in Design or Specifications of a registered medical device (GMD and IVD)
3.) Documentation Guidelines for Software Changes
4.) Changes to Materials in a General Medical Device
5.) Change to Materials of In Vitro Diagnostic Medical Devices
6.) Changes to Labelling
7.) Changes to registered medical devices listing information
Clarification on Change Notification (CN) document requirements
Additional remarks on some CN applications are found in Table 1.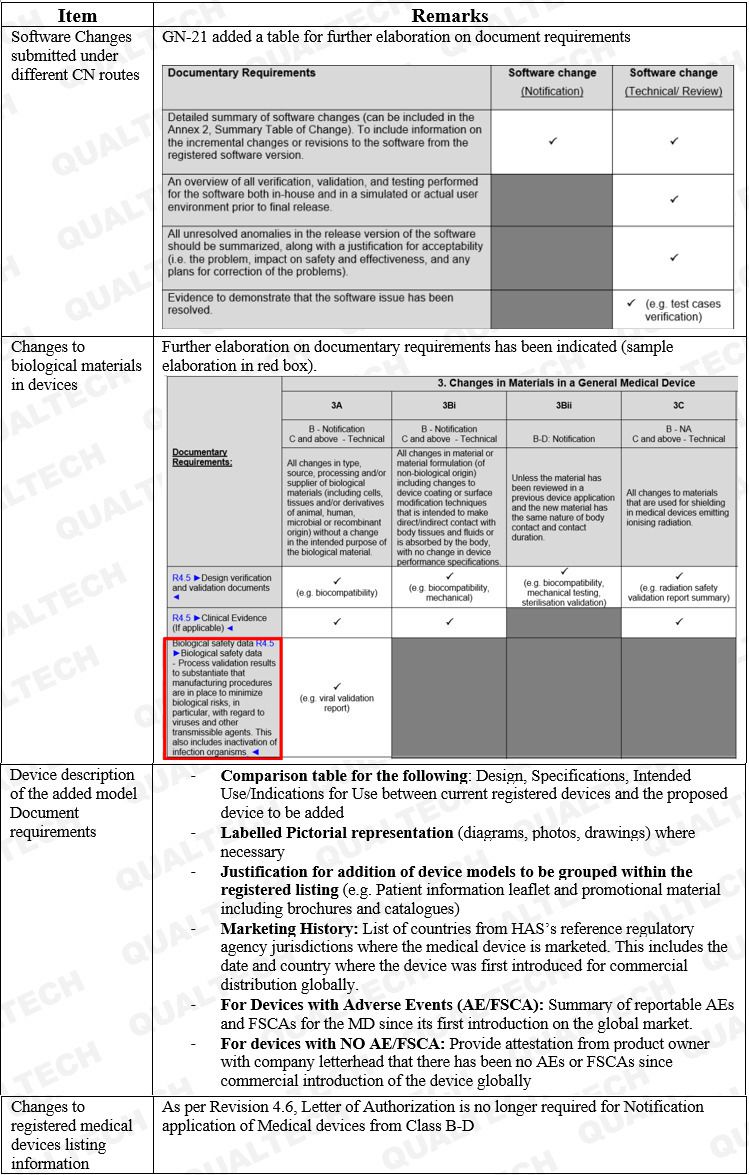

References:
Reference: Updates to GN-21: Change Notification of Registered Medical Device, GN-21: Guidance on Change Notification for Registered Medical Devices
