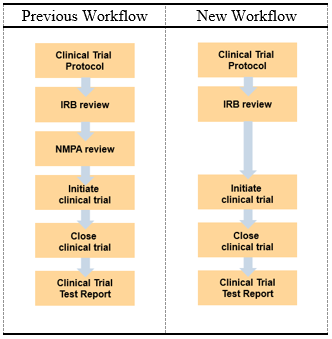
In order to optimize the approval procedures for the clinical trial, NMPA has made the following adjustments:
- Before submitting necessary documents in obtaining approval for conducting a clinical trial, the applicant may request an appointment for consultation with the “Medical Device Technology Review Center of National Medical Product Administration” (also referred to as CMDE). This is in accordance with the “Notice on matters about the communication and application of medical device clinical trials for approval” (CFDA No. 184, 2017)
- Within sixty (60) days upon receipt of the request and payment, the applicant may already proceed with the clinical trial if they have not received any feedback yet from CMDE (including the notice of the expert consultation meeting and the supplementary information notice).
- For those who were approved to carry out clinical trials, the CMDE shall publish the acceptance number, name and address of the applicant, product name, product model specification, structure and composition of the medical devices on website. Applicants shall be informed of the inspection results online instead of a hard copy notification.
Applicants are advised to check the CMDE website from time to time in order to check the status of clinical trial approval request.
Reference: