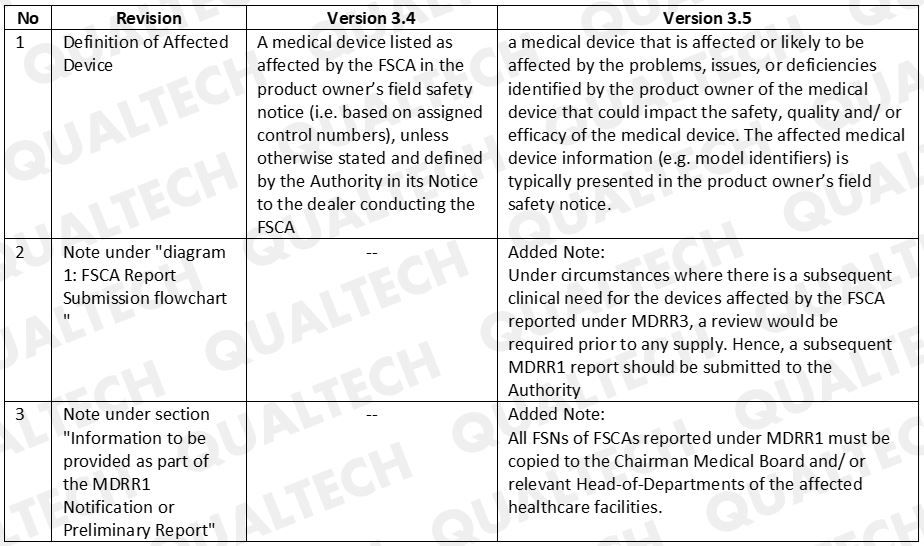
There were 3 revisions that HSA included in the latest GN-10 document. Refer to Table 1 to see the difference revisions added.
Table. 1 Latest revision for GN-10 is version 3.5 and it shows the following changes from the previous version
Implication of Changes
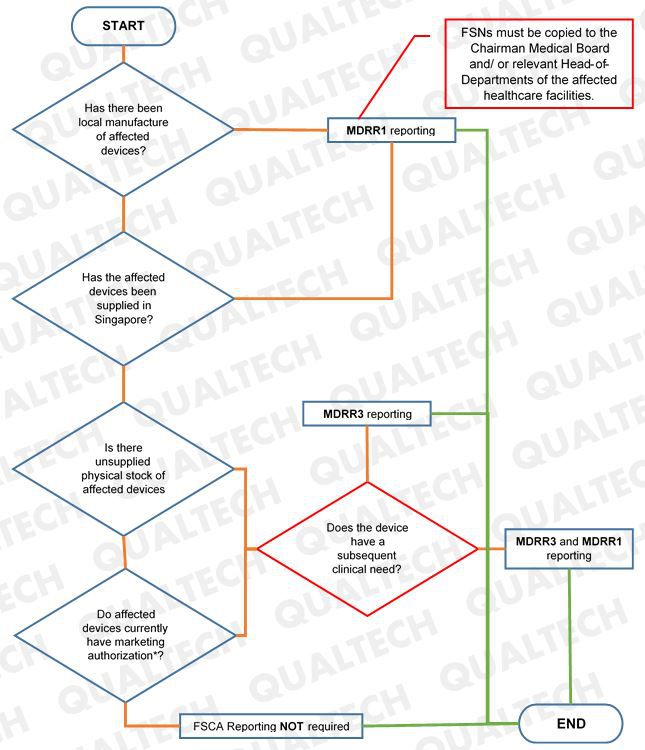
HSA clarifies that manufacturers who will do MDRR3 reporting for a device that is clinically needed in SG will need to undergo review and approval prior selling. In addition, manufacturer will also submit a MDRR1 report for the same device to HSA.
For FSCAs to be reported under MDRR1, FSNs are required to be copied to relevant officials of the affected healthcare facilities.
References:
Reference: Health Sciences Authority-Regulatory Guidance for GN-10