|
February 14, 2020
The Association of Southeast Asian Nations (ASEAN) consists of 10 rapidly developing countries in Southeast Asia. The medical device markets in this region and the respective governments offer a variety of opportunities and incentives for foreign medical device manufacturers. Among them is the gigantic market size of the region, consisting of a population of 622 million people (and thus bigger than the population of the entire European Union with 513 million people).
Below Qualtech has summarized the most convincing factors that make the ASEAN medical device markets extremely attractive and rewarding.
● 1) A Harmonized Regulatory Model – The ASEAN Medical Device Directive (AMDD)
In the past, the great variety in regulatory requirements for the different ASEAN countries have presented quite a challenge for foreign manufacturers. However, the ASEAN Medical Device Directive (AMDD) has made it significantly easier to penetrate the ASEAN markets collectively. The Directive
has the goal of harmonizing the medical device regulations and common technical documents, as well as establishing a common classification system based on the devices’ risks. In that way, technical barriers and regulatory hurdles are greatly reduced and manufacturers will be able to quickly approach the entirety of 622 million people of the ASEAN region.
Registrations will still have to be completed individually for each of the 10 ASEAN countries. Yet, with the AMDD, common regulations concerning post-market surveillance, quality control and a Common Submission Dossier Template (CSDT) are being implemented. Thus, this allows manufacturers to attain a faster market access to the various countries of the ASEAN region with drastically reduced costs.
The AMDD has been signed by all ASEAN member states and was ratified in 2017. Full implementation was scheduled for the year 2020, with some of the not yet compliant ASEAN Member States currently adjusting their national laws to be in line with AMDD.

Figure 1: Timeline of the ASEAN Medical Device Directive
● 2) A Sharp Increase in Demand for Medical Devices by A Rapidly Growing Middle Class:
ASEAN’s medical device markets are predicted to surpass many of the more well-established markets in the coming years. The steady rise of the ASEAN medical device sector is supported by
- a) a large and increasingly aging population (see item 3 below for further specifications),
- b) elevated government expenditures on healthcare (including universal healthcare systems and reimbursement schemes),
- c) and a significant growth of the middle class.
Main drivers of this growth had traditionally been countries such as Thailand, Singapore and Malaysia. However, based on recent observations from the past 5 years, other ASEAN nations - such as the Philippines and Indonesia - have seen higher growth rates of their respective medical device sales, averaging at around 10% annually.
Collectively, ASEAN’s medical device sales have amounted to US$ 5.3 billion in 2016. The number is however expected to reach US$ 8.5 billion next year according to the Business Monitor International (BMI) Database of 2018. Thus, projecting an enormous growth of 59% within a duration of only 5 years. Likewise, the Compound Annual Growth Rate (CAGR) for the same time period (2016 to 2021) is estimated at 9,7%. This in turn means that it will exceed the figures for markets such as India, Japan or the United States.
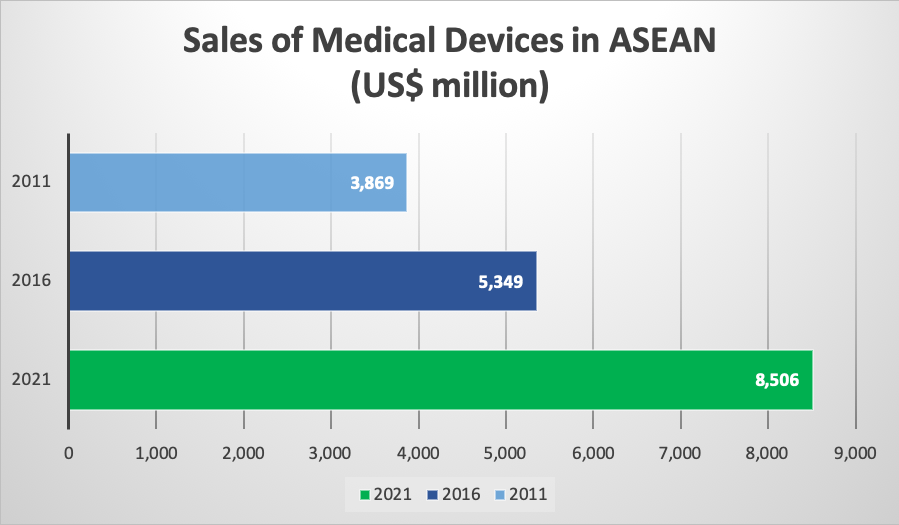
Figure 2: ASEAN Medical Device Sales - Business Monitor International (BMI) 2018
Further dramatic growth is expected for the future, due to an unprecedented rise in ASEAN’s middle class along with its increased disposable income for medical devices and healthcare related services. According to Bain and Company, Indonesia, Malaysia, the Philippines, Thailand and Vietnam alone will have additional 50 million new middle-class consumers by the year 2022.
Currently only around 44% of Indonesia’s population, 34% of the Philippine population, and 25% of the Vietnamese population are regarded as middle class and affluent consumers (MACs). Yet, there is a steady growth of MAC’s of over 5% annually in all three countries, which will further increase the already existing $300 billion middle-class disposable income of the ASEAN region. These new middle-class consumers, along with the existing ASEAN middle-class population are fueling the demand for quality healthcare services and treatment.
● 3) ASEAN’s Aging Population Will Further Spike the Need for Medical Devices and Eldercare:
Besides the major impact of ASEAN’s rising middle class, the region’s elderly population is also rapidly growing. In the year 2016, 39 million people in the ASEAN region were above the age of 65 (amounting to 6% of the total population). However, this number will nearly double to 58 million people over the course of the next five years (2025). Therefore, a greater need for medical devices and treatment, especially concerning eldercare services and related diseases is to be anticipated.
According to the most recent dataset available by the world bank from 2018, the highest percentage of elderly people in the ASEAN region are to be found in Thailand (12%), followed by Singapore (11%), Malaysia (7%), and Vietnam (7%). By the year 2050, the percentage of those over the age of 65 is further expected to triple to 123 million people. Singapore is predicted to lead the list with a remarkable number of 33.6% of its population being over the age of 65 by 2050.
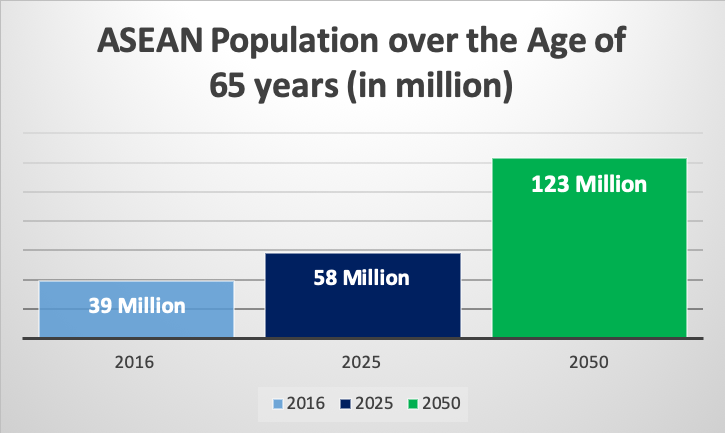
Figure 3: Rapidly Aging Population in ASEAN – BMI Database of 2018
Such a fast increase in the elderly population will present a massive financial burden on the respective local healthcare systems. At the same time, it will alter the medical landscape in the ASEAN countries with diseases such as cardiovascular disease, cancer, dementia, and diabetes becoming more and more common.
● 4) Local Medical Device Manufacturers Only Cover Less Than 10% of the ASEAN Market Needs:
One of the key opportunities for foreign manufacturers in the ASEAN region is the small competition from local medical device manufacturers. Many areas of the local medical device industry are not yet matured, especially the local production of high-tech medical device represents a challenge. Therefore, the ASEAN nations are heavily dependent on medical device imports.
In fact, more than 90% of the medical devices in the ASEAN region are imported. Main importing nations to Southeast Asia are the United States of America, Japan, as well as Germany. Thailand peaks the list in this respect, as a striking number of 97% of its medical devices consist of imports. However, foreign manufacturers shall note that highly advanced and costly devices may not be particularly suitable for the ASEAN markets, as they may be unaffordable for large parts of the ASEAN population.
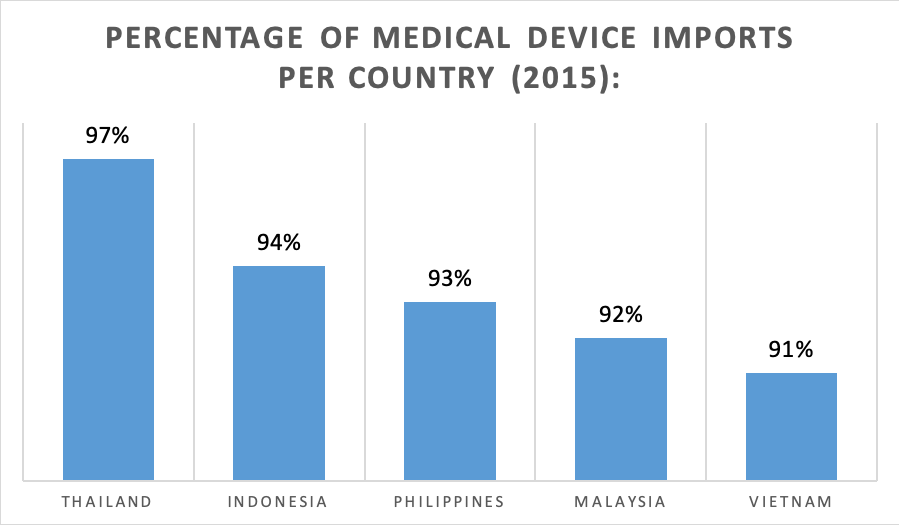
Figure 4: Medical Device Imports in ASEAN – Global Data 2018
While significant gaps can be identified within the local ASEAN medical device industry, there are however also several strong points. Local production of medical devices in the ASEAN region is dominated by consumables as well as medical furniture. Thailand, for instance, is globally known for its expertise in medical devices such as test kits, surgical gloves, and syringes. More recently, the country has also put significant efforts in developing its position as a medical tourism hotspot in the region. At the same time, foreign manufacturers continue to find ample opportunities in Thailand, while the Thai government is even encouraging foreign manufacturers to produce locally by having established corporate tax exemptions.
The figure below further highlights what type of medical devices are most frequently being imported into the ASEAN countries. Products currently on high demand are “Diagnostic active devices” and “IVDs”. Particularly high-tech implantable or consumable devices, such as dental implants, guidewires, and catheters are popular foreign products.
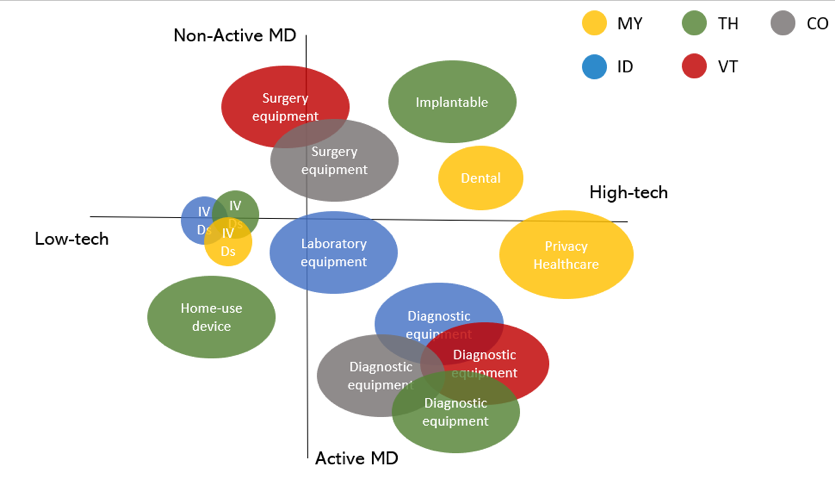
Figure 5: Types of Medical Devices Most Frequently Imported to ASEAN - Source: Export. Gov
● 5) ASEAN’s Well-developed Distribution Environment:
When talking about the ASEAN markets, it is also necessary to talk about distribution. Essential in this regard is to understand the requirements and regulations concerning the License holder. The role of the License Holder in the ASEAN countries may be designated to either the Importer, or an Authorized Representative (AR).
In the Philippines, Indonesia and Thailand, the importer assumes the responsibility of the License Holder. The Singaporean, Malaysian and Vietnamese market, on the other hand, require an AR to hold the license. ARs, in turn, can authorize other qualified distributors to perform the import procedures and to proceed with the distribution locally. In case our readers are interested in further relevant information, we kindly refer you to our previous article, which provides you with a comprehensive introduction of how the Asian nations’ governments regulate post-market surveillance.
● 6. Future Key Medical Device Segments for the ASEAN Markets:
Despite a general upward trend for the entirety of the medical device sector in ASEAN, experts of Global Data have identified cardiovascular, cancer, orthopedics, and ophthalmology devices as key growth areas in the ASEAN region.
With increasing cancer rates witnessed all over Southeast Asia, particularly Nasopharyngeal, lung, breast, liver, and colorectal cancer have become more common. Cardiovascular diseases on the other hard represent the leading cause of death in Southeast Asia. Indonesia; for instance, has seen an average increase of its annual mortality rate from cardiovascular diseases by 1,9% each single year for the past 25 years. Thus, representing one of the fastest growing medical device sectors in the country and the entire region alike.
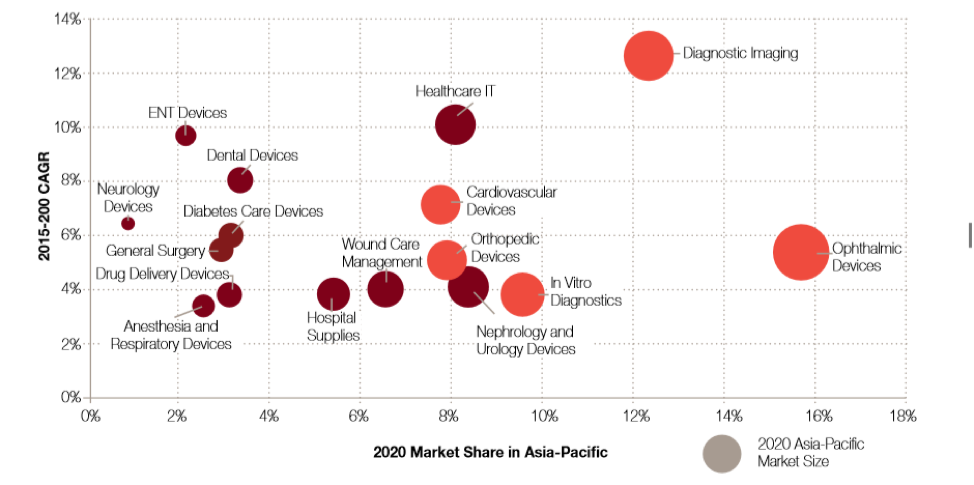
Figure 6: Growth of Medical Device Segments – Global Data 2018
Furthermore, the need for orthopedic devices, such as those for treating osteoporosis, are on the rise in Southeast Asia. According to the information collected by Global Data, orthopedic devices will have grown 5,1% every year during the period of 2015 to 2020. Other medical device segments that are expected to see significant future growth also include, ENT devices, neurology devices, diabetes care devices as well as dental devices.
● ASEAN – Your Opportunity:
The ASEAN region represents a very attractive and rewarding market for foreign medical devices manufacturers. The demand for medical devices is steadily increasing, the number of imports is remarkably high, all the while ASEAN’s population is significantly growing in size, disposable income as well as in age.
Qualtech, with more than 20 years of experience in the Asian markets, represents the ideal partner for tapping into the thriving markets of Southeast Asia. Operating through our local Qualtech teams and branches in the different ASEAN countries, we are not only familiar with all regulatory aspects, but also with the existing diversity among the ASEAN nations, including different cultures, languages, and patient beliefs. Shall you be interested in expanding your market reach into Southeast Asia, please feel free to email us: irene@qualtechs.com.
References:
• ASEAN Medical Device Directive in English Language
• Qualtech’s Analysis of Singapore, Malaysia, Thailand and China’s Government Expenditures for Healthcare
• Report by Bain & Company” “Understanding Southeast Asia’s Emerging Middle Class”
• World Bank Data from 2018 concerning the “Population ages 65 and above”
• Export gov: Healthcare resource guide
• International Trade Administration
• PwC Growth Markets Centre – The Future of ASEAN (May 2018)
Tags:
Qualtech Analysis, ASEAN, Market Information
|






