The Government of India, Ministry of Health and Family Welfare has released guidelines with respect to Grouping of Medical Devices for applicants who intend to apply for a license to import or manufacture medical devices for sale and distribution in India.
General guidelines are hereby summarized as follows:
- Application for license to import or manufacture of medical devices shall be carried out as specified in the provisions given in the respective forms found in the Appendices of Medical Devices Rules 2017
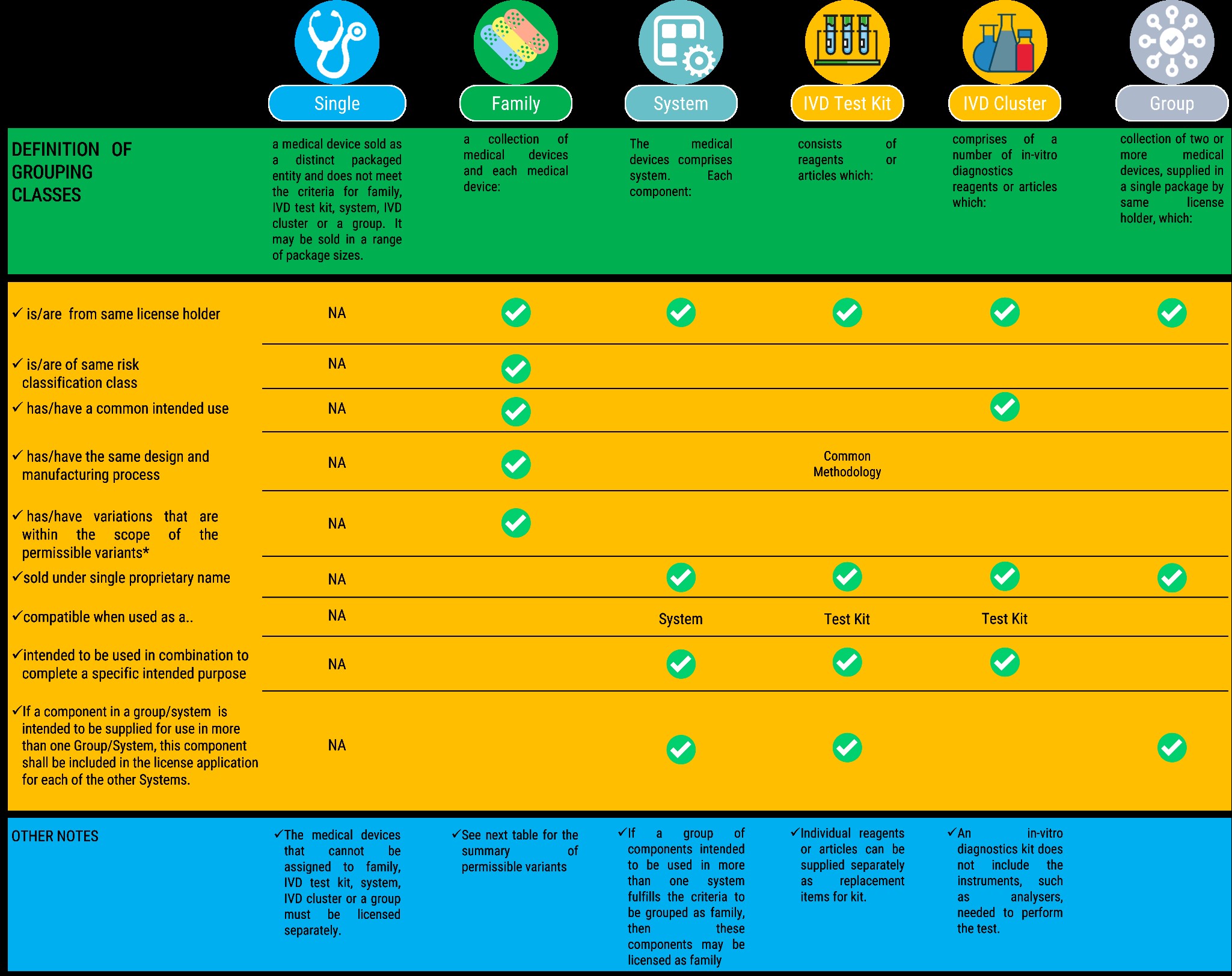
- Medical devices with same or similar intended uses or commonality of technology may be grouped together and submitted as a single application.
Medical Device Grouping shall have six (6) categories, namely (a) Single (b) Family (c) In vitro diagnostics Test Kit (d) System (e) In vitro diagnostics cluster (f) Group
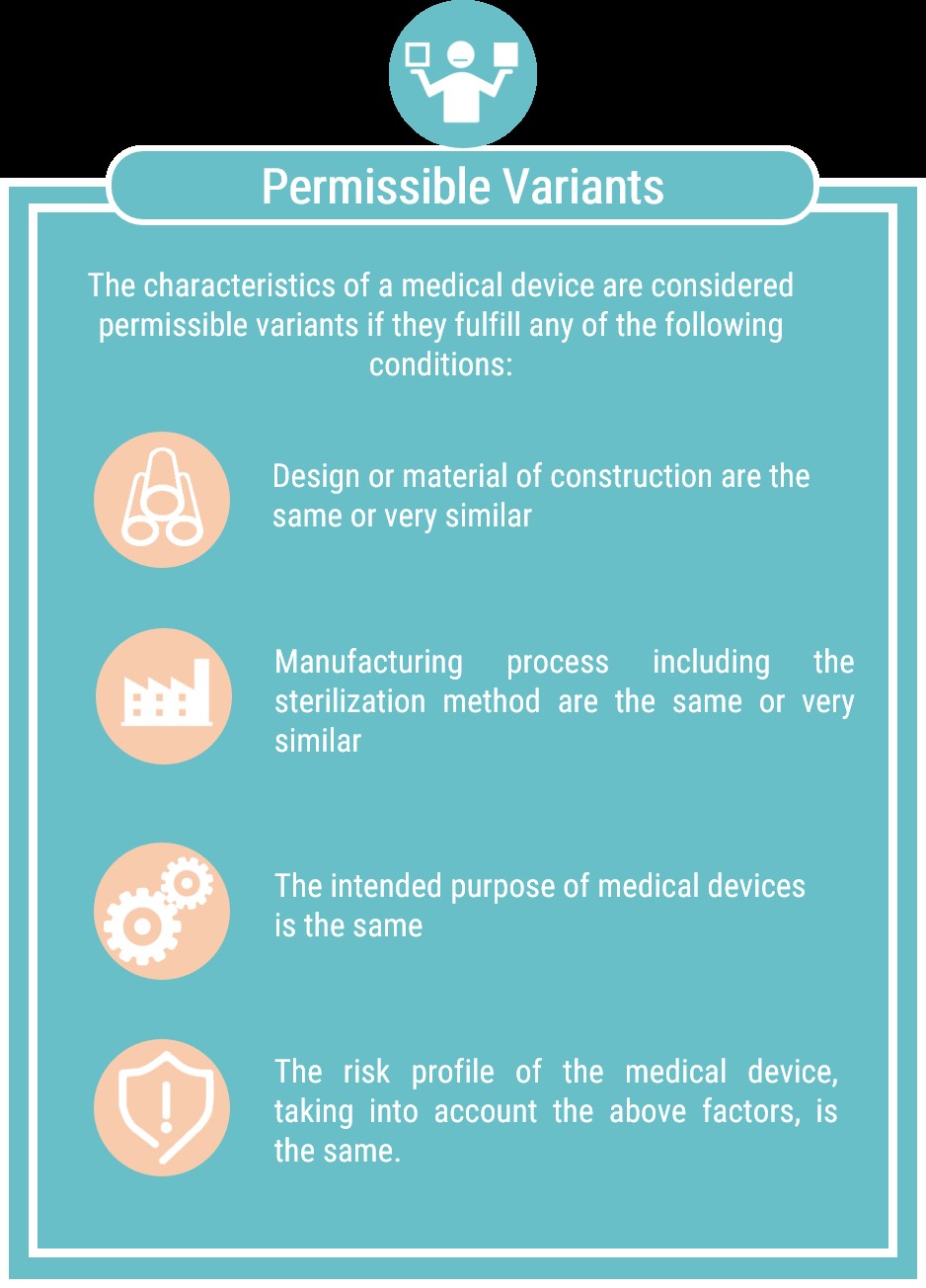
The specific rules for grouping medical devices are summarized in the tables that follow:
Reference:
Guidelines on Medical Device Grouping for License Application
