New amendments on the upcoming Medical Device Regulations have been drafted out just as a preliminary Guidance on Labeling was released in early May. The AO 2018-0002, which was supposedly implemented last April 11, has been postponed due to some changes.
Details of changes are as follows:
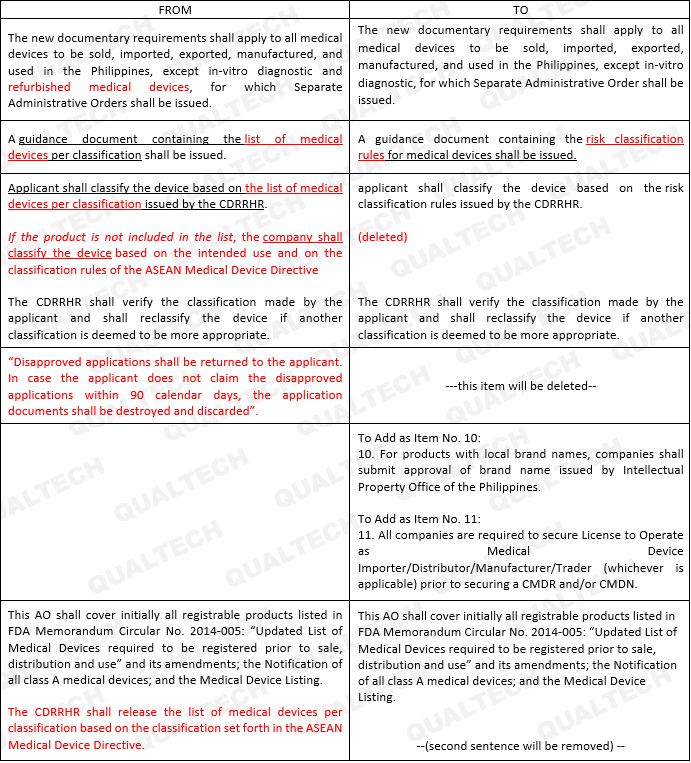
In this update, it is noteworthy to keep in mind that product classifications shall be made by the applicant. Rules for Classification are more likely to follow the Rules set by the ASEAN Medical Device Directive (AMDD).
Other amendments to take note of are the inclusion of refurbished medical devices in this guideline and requirements for licensing local products.
At the time of this writing, no further updates on the implementation date is yet to be announced. However, clients are expected to prepare the necessary documentations, most especially for Registrable Medical Devices. From the previous issues, it could be recalled that once the implementation of the AO kicks in, documentary requirements for Registrable Medical devices should follow the CSDT format.
As PFDA is starting to prepare for the implementation of the new Medical Device Regulation, more changes should be expected. Hence applicants and clients alike should keep a close watch for more updates.
References:
Reference: Amendment to Administrative Order No. 2018-0002 dated 26 January 2018, re: Guidelines Governing the Issuance of an Authorization for a Medical Device based on the ASEAN Harmonized Technical Requirements
