NMPA plans to promote the use of its online submission system for medical device registration (eRPS system). In order to protect the security of the registrants’ accounts, NMPA requires medical device manufacturers and legal agents to apply for a so-called Certificate Authority (CA) certificate for the eRPS system. The relevant matters concerning the CA application are as follows:
From May 10, 2019, the registrant can apply for the CA certificate through the eRPS system (electronic Regulated Product Submission). A CA certificate represents a digital certificate specifically for NMPA’s new electronic registration platform. The certificate holder shall be the domestic manufacturing enterprise of Class III medical device or the respective China agent of the imported medical devices of the foreign manufacturing enterprise. The online submission; however, does not change the original registration acceptance process, which still covers an administrative review as well as a technical review. Each company is further only required to apply for one CA certificate with signature function, while every CA certificate will be valid for a duration of one year.
From June 24, 2019, the online submission system will be implemented. China agent shall possess CA certificate to have capacity to submit the registration application. It’s important to take care if your China agent gets the CA certificate.
The process for the initial application of the CA certificate:
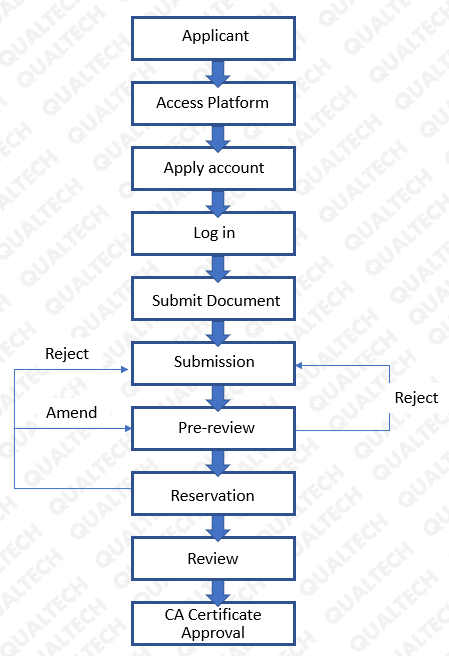
Reference:
