August 13, 2018
Health Sciences Authority (HSA) monitors all health products, including medical devices, to assure of their safety and efficacy prior entering the Singapore market. The process of registration, licensing, notification and permit issuance to medical devices involves operation costs that are charged via fees upon submission of any application to HSA.
It’s been a decade since the existing fees are changed going back to 2016. The surge in operating expenses over the last decade has resulted for a change of existing fees to cover the part of these expenses for the existing services.
To minimize impact on industry stakeholders, a minimum increase of $1 will be applied for low fee items. For complex regulatory review and processes, the increase will be capped at $200. Listed in the table below are all the fees that will be implemented by April 2, 2019:
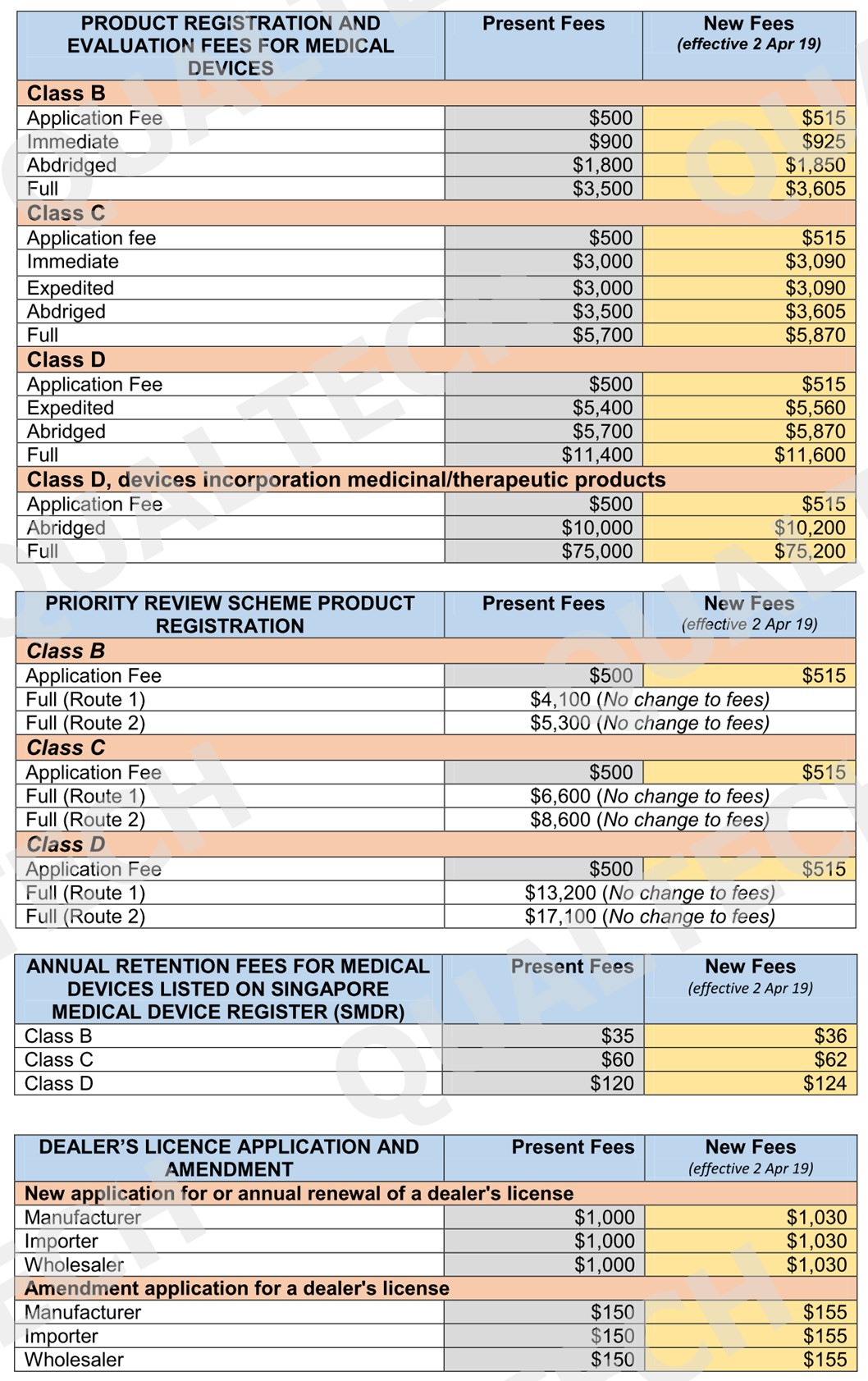
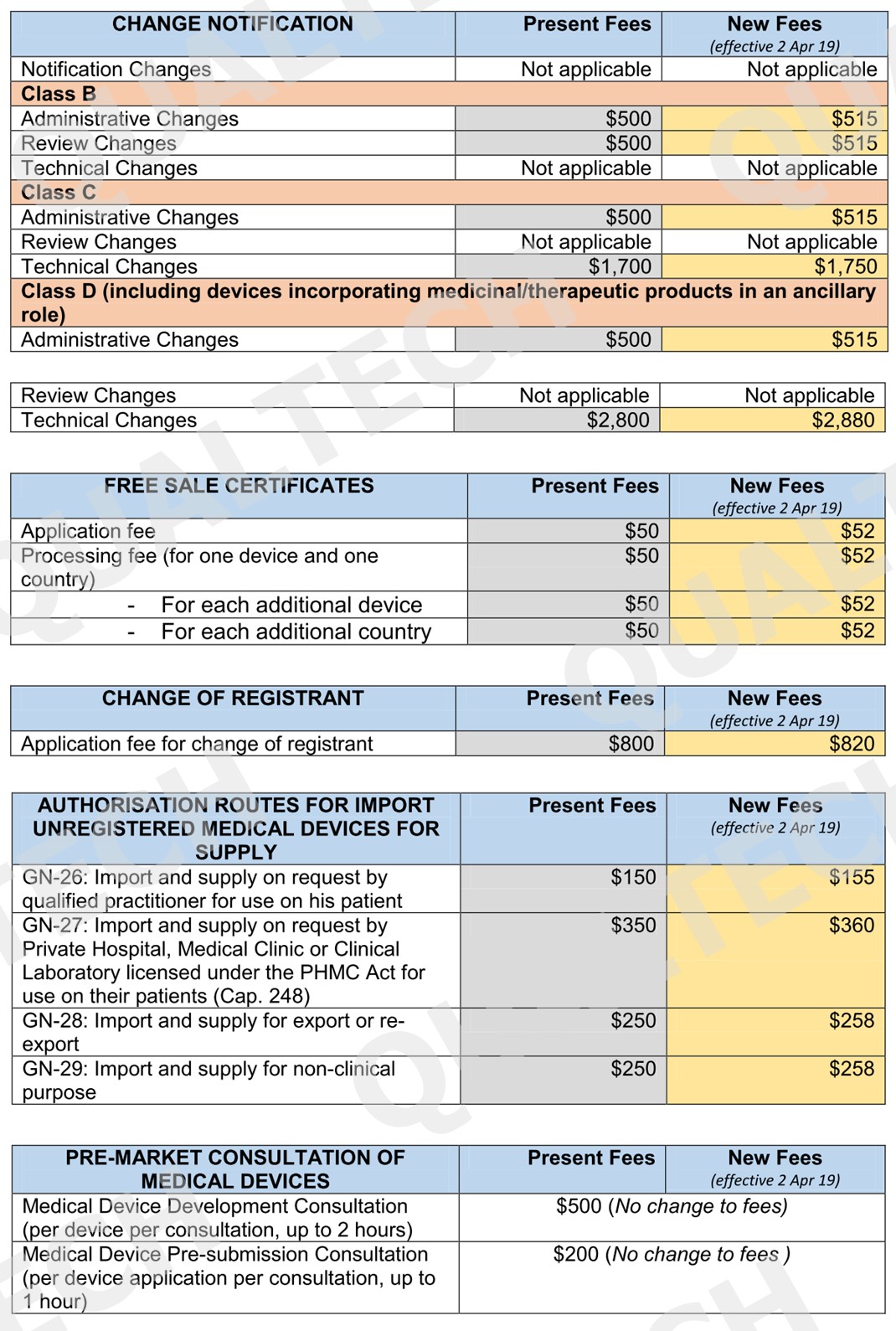
Conclusion
This coming April 2019, new fees for HSA regulatory processes will be implemented in order to maintain the regulatory efficiency of the agency without sacrificing regulatory control. Industry stakeholders at this time are encouraged to be familiar with these changes before timely implementation.
Reference:
List of New Fees for Health Products [HSA] - Effective 2 April 2019
