The medical device market in Vietnam is one of the fastest growing in the Asian region due to the government's recent hospital improvement efforts. More than 90% of medical devices in Vietnam are imported. Qualtech is your ideal partner for your foray into the Vietnam medical device market for an easy and smooth registration process.

Qualtech in Vietnam
Expert in Non-Active Device
Our team has assisted with various kinds of medical devices to access Vietnam market efficiently. Our experience covers contact lens, facial filler, adhesive gel, sterilizers and consumable products, cranial saw drilling system, and other devices.
Market Access Strategy
Qualtech Vietnam team offer clients ONE-STOP market access service, covering Regulatory research, Registration, Importation, Distributor connection.

Medical Device Registration
Our in – house experts are able to provide you with excellent professional service in preparing customized ASEAN Common Submission Dossier Template (CSDT) and liaise with IMDA officers to get your devices registered in the Vietnam.

Authorized Representation
As an in – country authorized representative (AR), Qualtech can hold a medical device registration license on behalf of foreign manufacturers looking to market medical devices in Vietnam. This is in compliance with the law for a local establishment to be a license holder.
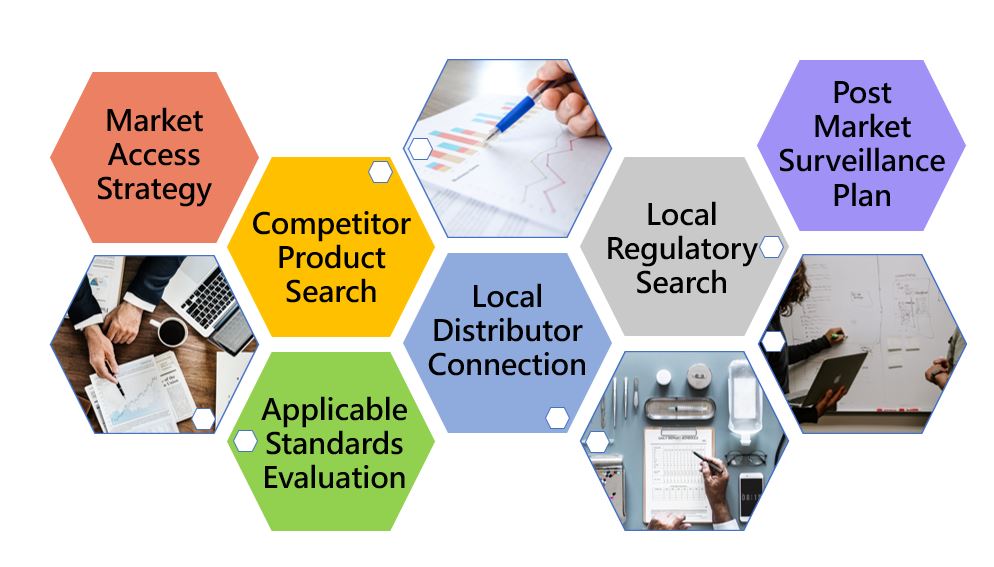
Importation
The local distributor you are looking at is sometimes not a qualified importer, or is not familiar with the importation process of medical devices. Qualtech has extensive experience in importation in many regions of Asia, assisting several customers to handle customs clearance of various medical equipment imports. We can assist your products be delivered to customers smoothly!
| Registration with IMDA | |
|
 |
CLASS A | CLASS B | CLASS C | CLASS D |
|---|---|---|---|---|
| Executive Summary | Yes | Yes | ||
| Essential Principles and Methods | Yes | Yes | ||
| Device Description | Yes | Yes | Yes | Yes |
| Summary of Design V&V | Yes | Yes | ||
| Labeling and IFU | Yes | Yes | Yes | Yes |
| Risk Assessment | Yes | Yes | ||
| Physical Manufacturer Information | Yes | Yes | Yes | Yes |
| Summary of clinically testing data | Yes | Yes | ||
| PMS Report | Yes | Yes | ||
| Declaration of Conformity | Yes | Yes |
Note: Kindly see the Decree 98/2021/ND-CP and Decree 07/2023/ND-CP for more details. From 2021, Class C and Class D medical device registration in Vietnam will not require CSDT until 2024. The CSDT must be applied as of January 1st, 2024.
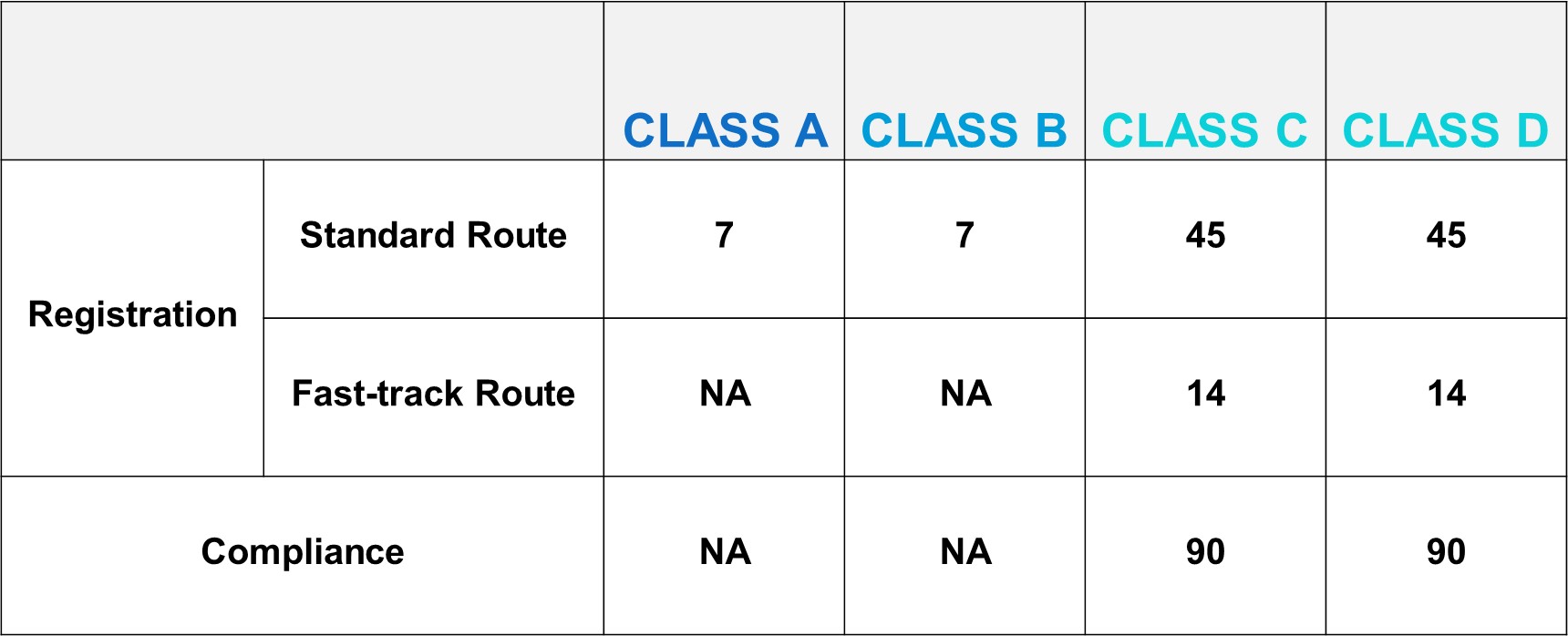
DoH/IMDA Target Reviewing Evaluation Time
After successful internal evaluation of the submission dossier, our in – house experts will then submit it to the DoH/IMDA and will be subjected to thorough evaluation. Below is a list of the evaluation times for different types of transactions involving product registration/notification.
-
-
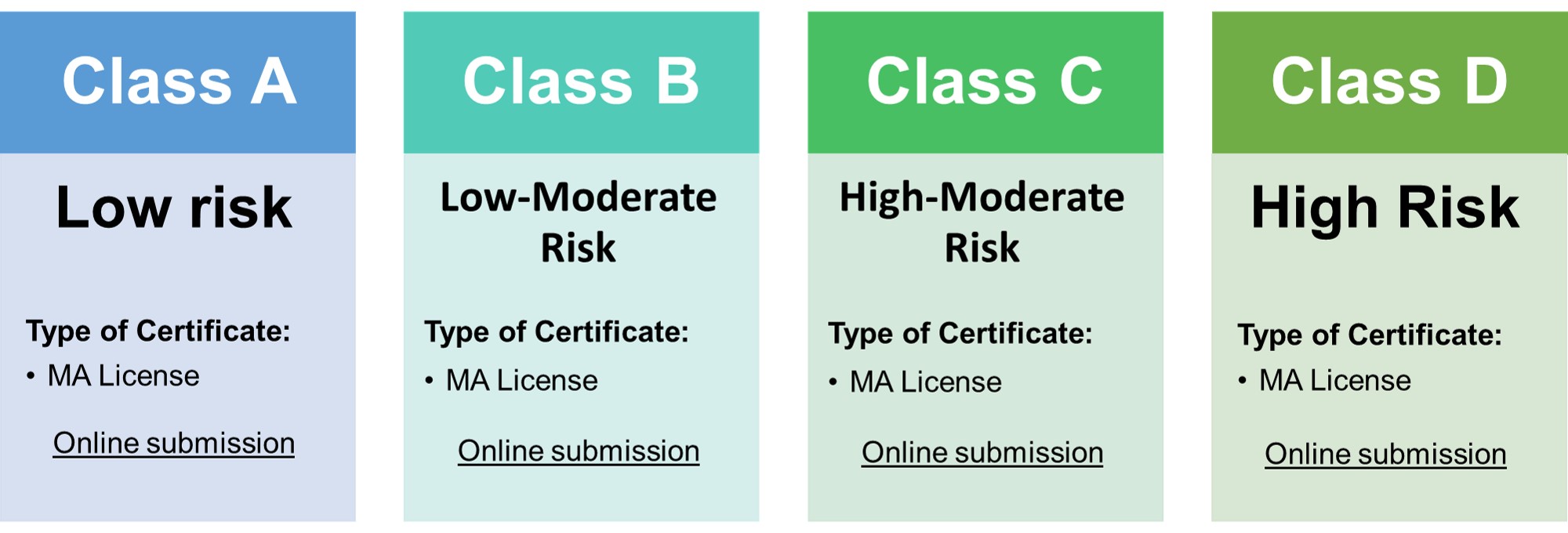
Types of Certificates:
-
-
• Class A & B: Declaration of applicable standards for medical device
- • Class C & D: Certificate of registration for circulation
- Validity of product certificate of class A, B, C, D: Permanent
-
According to the new regulation, the threshold of accessing Vietnam market becomes higher. Every foreign manufacturer would like to have their product access Vietnam market immediately. The good news is that IMDA offers TWO routes of faster market access to shorten the registration timeline.
The product has been registered in one of the following countries: Japan, Canada, Australia, United States, EU member countries, Switzerland, England, China, and Korea.
The medical device has been granted import licenses, registration numbers, or certificates of registration in commercial form in Vietnam, unless the certificate is revoked.
For more information, please refer to the Vietnam IMDA's official website or you may contact us for a free consultation.
We collect your browsing history through cookies to understand how you use our website to analyze and improve your experience. By continuing to use our website, you accept our use of cookies.
