Healthcare is a priority in Indonesia's national development agenda. Hence, it presents excellent opportunities for foreign exporters of medical equipment and supplies. Qualtech is your ideal partner for your foray into Indonesia's market of medical device with an easy and smooth registration process.

Qualtech in Indonesia
IDAK Certificate
We are an approved entity by the Government of Indonesia, holding our IDAK Certificate (Izin Distribusi Alat Kesehatan / Medical Device Distributor License), to process the regulatory and distribution work of medical devices. We have successfully assisted many foreign manufacturers to access Indonesia’s local market.
CDAKB – GDP (Good Distribution Practice)
Indonesia’s Ministry of Health (MoH) have decreed that in order to ensure the quality of medical devices regulated in Indonesia, Good Distribution Practice (GDP) or Cara Distribusi Alat Kesehatan yang Baik (CDAKB) must be conducted. We, in Qualtech, have implemented this standard in our work.
Expert in All Medical Devices
We offer clients an efficient registration way for all medical devices, as we are experienced in bringing products varying in risk levels, low or high, to the open local market in a short time. We have obtained more than 100 cases’ approval.

Medical Device Registration
Our in – house experts are able to provide you with excellent professional service in preparing customized ASEAN Common Submission Dossier Template (CSDT) and liaise with MoH officers to get your devices registered in the Indonesia.

Authorized Representation
As an in – country Authorized Representative (AR), Qualtech can hold a medical device registration license on behalf of foreign manufacturers looking to market medical devices in Indonesia. The following is in compliance with the law for a local establishment to be set as a license holder.
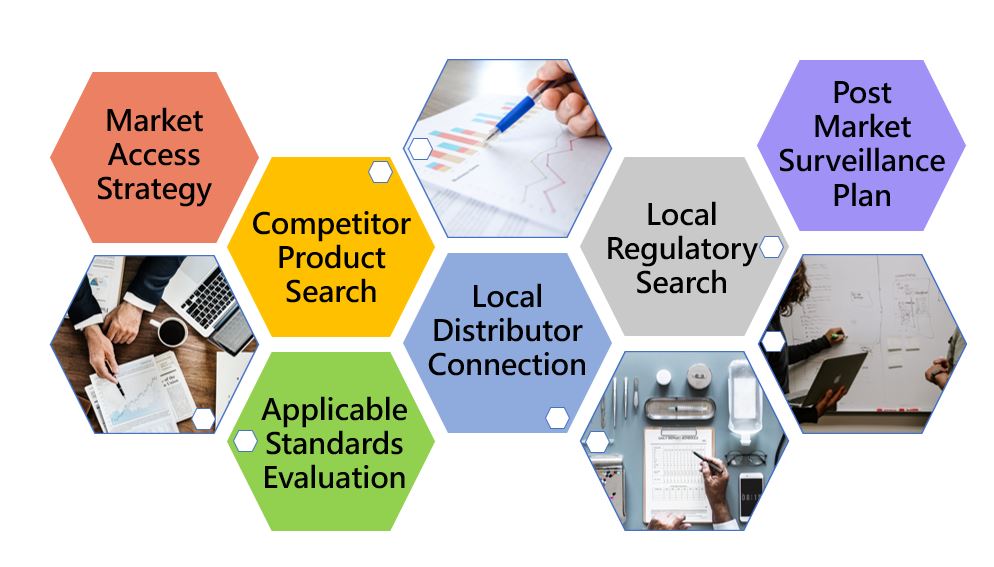
Importation
Qualtech has extensive experience in importation to many regions of Asia, assisting customers to handle custom clearances of various medical equipment imports. We can assist your products so they may be delivered to your customers smoothly!

E-katalog Application
We also provide professional guidance and service for local manufacturers, importers, distributors and authorized representatives to register and feature their products in E-katalog, the official site for the procurement of products and service providers. Having a team of local consultants who are well-versed in the requirements of local regulations, your journey will be smooth with us.
|
Registration with MoH |
|
|
 |
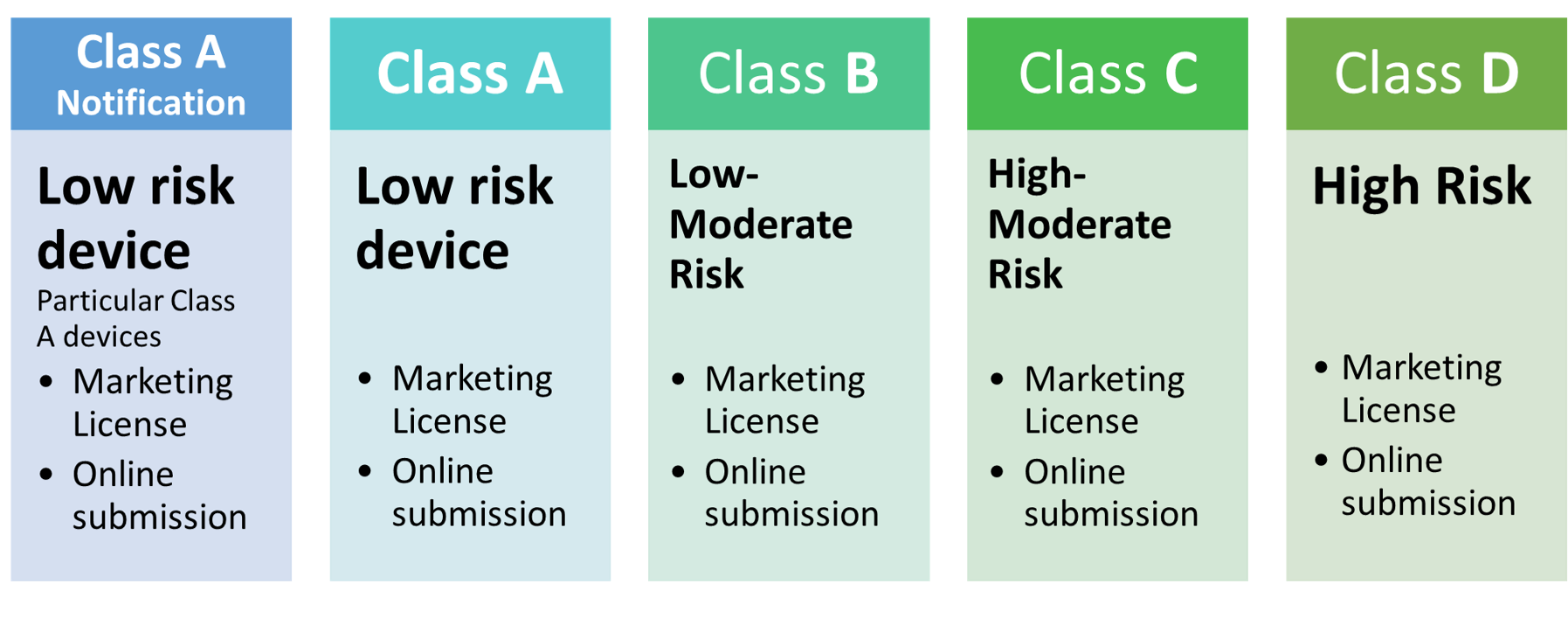
CLASS A Notification | CLASS A | CLASS B | CLASS C | CLASS D |
|---|---|---|---|---|---|
| Executive Summary | Yes | Yes | |||
| Essential Principles and Methods | Yes | Yes | Yes | ||
| Device Description | Yes | Yes | Yes | Yes | Yes |
| Summary of Design V&V | Yes | Yes | Yes | Yes | |
| Labeling and IFU | Yes | Yes | Yes | Yes | Yes |
| Risk Assessment | Yes | Yes | |||
| Physical Manufacturer Information | Yes | Yes | Yes | Yes | Yes |
| Clinical Evidence | Yes | Yes | |||
| PMS Plan and Report | Yes | Yes | Yes |
Note: Kindly see the Guidelines for Evaluation of Medical Devices According to The Regulation of MoH Number 62/2017 issued on 2019 for more details.
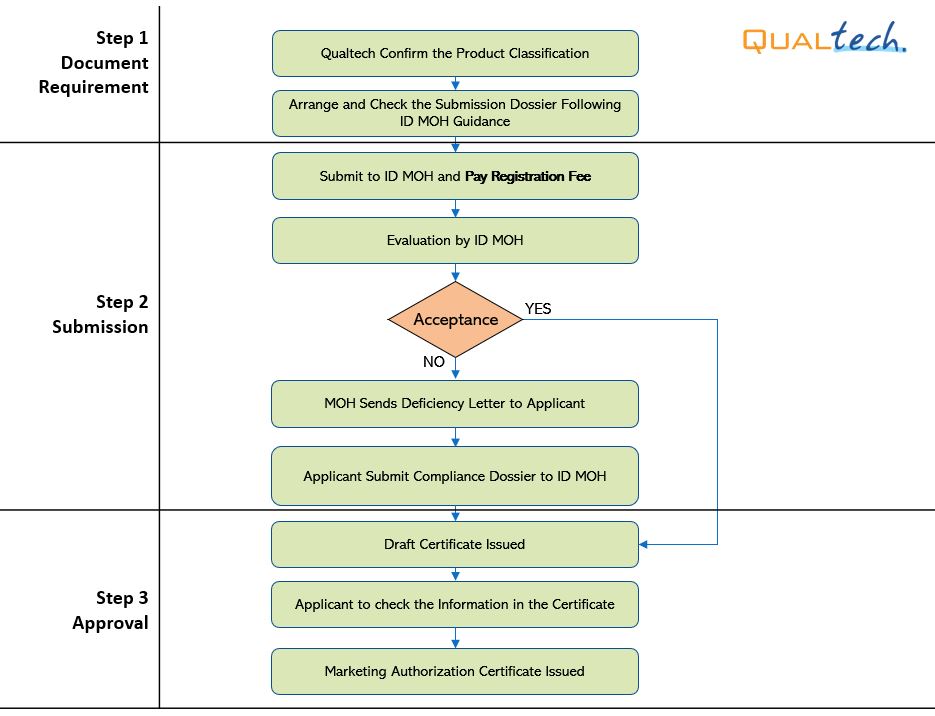
MoH Target Reviewing Evaluation Time
After successful internal evaluation of the submission dossier, our in – house experts will then submit it to the MoH and will be subjected to thorough evaluation. Below is a list of the evaluation times for different types of transactions involving product registration/notification.
|
CLASS A Notification |
CLASS A |
CLASS B |
CLASS C |
CLASS D |
|
|
1st Evaluation |
6 |
15 |
30 |
30 |
45 |
|
Compliance |
10 |
10 |
10 |
10 |
15 |
|
2nd Evaluation |
6 |
10 |
10 |
10 |
10 |
- Note:
- 1. It's accounted as Working days.
- 2. There is only one opportunity to comply with evaluator's additional input requests.
-
Types of Certificates: Marketing License
-
Marketing License: Depends on the validity of LOA, with 5 years as the maximum
Some manufacturers might face poor selling performance in Indonesia and would like to change their sole distributor. We have received several inquiries such as these from our clients or partners.
Unfortunately, it’s a hard task to change unto another local agent in Indonesia because of law restrictions – MoH requests the no objection letter from the current local agent. Usually, the local authorization representative would be unwilling to issue this letter.
To avoid this situation from your sales work in Indonesia, we would suggest putting your license in a 3rd party, such as Qualtech.
For more information, please refer to the Regulation of MoH Number 62/2017 or you may contact us for a free consultation.
- MoH Product Database
- MoH Registration Website & Announcement Board
- MoH Post Market Surveillance System
We collect your browsing history through cookies to understand how you use our website to analyze and improve your experience. By continuing to use our website, you accept our use of cookies.
