OUTLINE
- Overview of Healthcare in Indonesia
- Medical Device Market in Indonesia
- Importation of Medical Devices in Indonesia
Indonesia is the world’s fourth most populous country. Supporting the United Nations (UN) Millennium Development Goals (MDGs), the Indonesian government released their national development plan (RPJMN 2015, Propenas) in an effort to reduce poverty. Healthcare is one of the top priorities in Indonesia’s national development agenda.
Overview of Healthcare in Indonesia
Indonesia presents excellent opportunities for exporters of medical devices and supplies. The government continues to build hospitals and upgrade healthcare facilities. There are also plans to extend the upgrade of in-patient facilities and quality of services up to the community health centers.
“Indonesia began implementing its Universal Health Coverage (UHC) Plan in 2014, locally known as Jaminan Kesehatan Nasiona or JKN Program (National Health Insurance System), with the goal of universal coverage for the country’s population by 2019.” This opens up a strong increase in the demand for medical devices and services, but is being challenged by “underfinancing, shortage of primary healthcare providers and hospitals, limited access to drugs in other rural areas, and overall inaccessibility and inequity of care.” According to Business Monitor International (BMI), because of the increasing population in the country, the UHC will be forced to expand in the coming years, resulting to the proper re-allocation of funds and resources to make their healthcare system meet global standards.
Medical Device Market in Indonesia
As a member of the ASEAN community, Indonesia is one of the “most attractive emerging markets in the world for business development.” The Indonesian medical device market is projected to rise by a Compound Annual Growth Rate (CAGR) of 12.7%, which ranks it amongst the top 15 fastest growing markets in the world. With this growth rate and a lack of domestic manufacturers, there are a lot of opportunities for profit for foreign manufacturers who are willing to market their products in Indonesia.
Table 1. Projected Medical Devices (2014 to 2019)
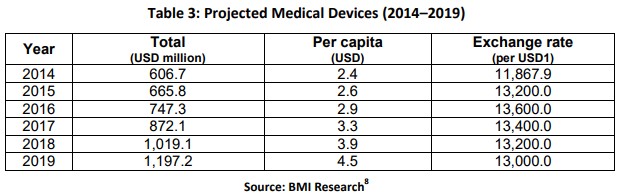
Indonesia still imports 97% of its medical devices as of 2014. “Local companies produce only basic medical equipment, like surgical gloves, bandages, orthopedic aids, and hospital furniture. A breakdown of the market shows that 35% consists of diagnostic imaging products, 23.8% of medical consumable products, 5.7% of auxiliary devices, 2.2% of dental equipment, 2.2% of orthopedic implants, and 31.2% are other types of medical devices.”
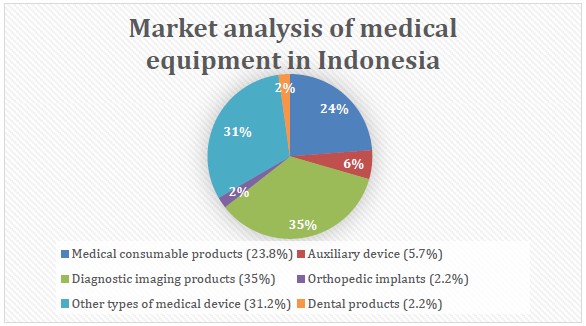
Figure 1. Market analysis of medical equipment in Indonesia. Source: Cekindo as cited by EIBN.
Currently, the country still spends lower than the global average for its healthcare spending. However, since implementing the UHC and JKN programs, there has been a steady increase in the total expenditure on health. This has a positive implication to the healthcare plan of Indonesia, as well as the manufacturers who are investing and planning to invest in medical devices in the country.
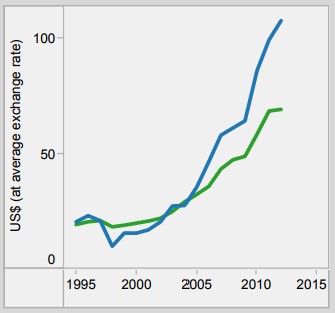
Figure 2. Per capita total expenditure on health. (Source: WHO)
In summary, because the National Health Insurance System will grow the demand for advanced healthcare equipment and medical devices, analysts expect that there will be exponential growth in the country's healthcare industry in the following years. Hence, a lot of opportunities for local companies and foreign manufacturers will open up as the demand continue to increase.
Importation of Medical Devices in Indonesia
Medical devices in Indonesia are regulated by the Ministry of Health of the Republic Indonesia (MOH), The Directorate General of Pharmaceutical Services & Medical Devices. MOH regulates the distribution of medical device in Regulation of the Ministry of Health of the Republic of Indonesia No. 1191/MENKES/PER/VIII/2010 where according to Article 9 company who will distribute a medical device in Indonesia should be a local entity company who possess a Medical Device Distributor License (IPAK) which issued by the Indonesia MOH. According to Regulation of the Minister of Health of the Republic of Indonesia No. 1190/MENKES/PER/VIII/2010 in Article 5, Medical Devices which will be imported, used and/or marketed within the territory of the Republic of Indonesia, should first possess the marketing license where it is issued by MOH.
Regarding the importation which is regulated in the Regulation of Minister of Trade of Republic of Indonesia No. 70/M-DAG/PER/9/2015 Article 2, importation can only be conducted by an importer that possess an Import Identification Number (API) where it is issued by the Indonesia Ministry of Trade. Besides having an API, according to the Regulation of Director General of Custom Clearance No. Per-04/BC/2017 Article 2, company who wants to import to Indonesia need to do a registration to the Director General Custom Clearance to be given an access to do the custom activity.
In addition to the importation, since December 2008 Indonesia has adopted an electronic system, Indonesia National Single Window (INSW). INSW is a national system that integrates all entities related to customs release and clearance of cargoes with the aim of accelerating the settlement process of import-export services and increased effectiveness and performance of traffic handling import-export goods. Information such as registration number, HS code number, tax number and country of origin are transmitted into INSW.
Foreign manufacturers who are importing medical devices to Indonesia must authorize a local company as their Authorized Representative (AR). It can either be a subsidiary office in Indonesia or other local medical device distributor, as a sole distributor/sole agent to register the product to Indonesia MOH. In addition, the manufacturer should ensure that the AR already possesses Medical Device Distributor License (IPAK), Import Identification Number (API), and registered to the Director General Custom Clearance.
Qualtech is Your Way into the Indonesian Medical Device Market
Qualtech has obtained GDPMD and IPAK (distribution license) in this year. We intend to provide you not only professional and efficient medical device regulatory consultation but a strategic solution by acting as your local representative. We are ready to be your best partner to enter Indonesia market and grow your business. Are you ready?
References:
Part I:
(1) Indonesia Country Commercial Guide – Medical Equipment
(2) EIBN Sector Reports – Medical Devices (Indonesia)
(3) Indonesia: WHO Statistical Profile
(4) Indonesia Medical Devices Report Q2 2015
Part II:
(1) Regulation of the Minister of Health of the Republic of Indonesia No. 1191/MENKES/PER/VIII/2010
(2) Regulation of the Minister of Health of the Republic of Indonesia No. 1190/MENKES/PER/VIII/2010
(3) Regulation of Minister of Trade of Republic of Indonesia No. 70/M-DAG/PER/9/2015
(4) Regulation of Director General of Custom Clearance No. Per-04/BC/2017
(5) Medical Devices regulatory systems: WHO
(6) Overview of Medical Devices Market in Indonesia
