
The Thai medical device market in the past years has been greatly supported by the advantages of its service quality and standard. The policy for a Medical Hub, which the Thai government has announced since 2003, has resulted in a gradually growing Medical Tourism industry. Qualtech is your ideal partner to for your foray into the Thailand medical device market for an easy and smooth registration process.

Qualtech in Thailand
Establishment Importer License
We are Thailand Government approved entity to process regulatory and distribution work of medical device. We successfully assisted foreign manufacturers to access local market.
Local Market Access
Qualtech Thailand team offers you ONE-STOP market access service, covering Regulatory research, Product diagnostic, Registration, Importation, Distributor connection.

Medical Device Registration
We can provide you excellent step-by-step professional service in compiling a submission dossier, known as the CSDT and submit on Thai Food and Drug Administration (Thai FDA) Medical Device Control Division, and follow up and liaise with the authorities to get your medical devices registered in Thailand.

Authorized Representation
Only companies which hold Establishment Importer License can be the local agent in Thailand. Qualtech’s service includes holding the license or CPR on behalf of foreign manufacturers seeking and aiming to market medical devices in Thailand. Furthermore, we are your bridge to Thai FDA and Thai Custom.

Medical Device Importation
Qualtech can supervise the successful import and custom clearance of your medical devices and provide full marketing support. We establish an experienced team to process custom clearance and ensure your products can be shipped to the distributor in safety.
Overview of Thai FDA Regulation
Medical devices in Thailand are regulated by a specialized division in the Thai FDA. The Medical Devices Control Division ensures that medical devices are wisely regulated and meet safety, quality, and efficacy standards. Submission dossiers are required to be prepared according to the harmonised CSDT guideline.
Only local representative holding an Establishment Importer License is allowed to make medical device registration with Thai FDA. There is no exclusive agent concept and no limitation is imposed to the quantities of devices for 1 Establishment Importer License holder as long as the “Specialty” scope on the license covered the UMDNS code of imported product.
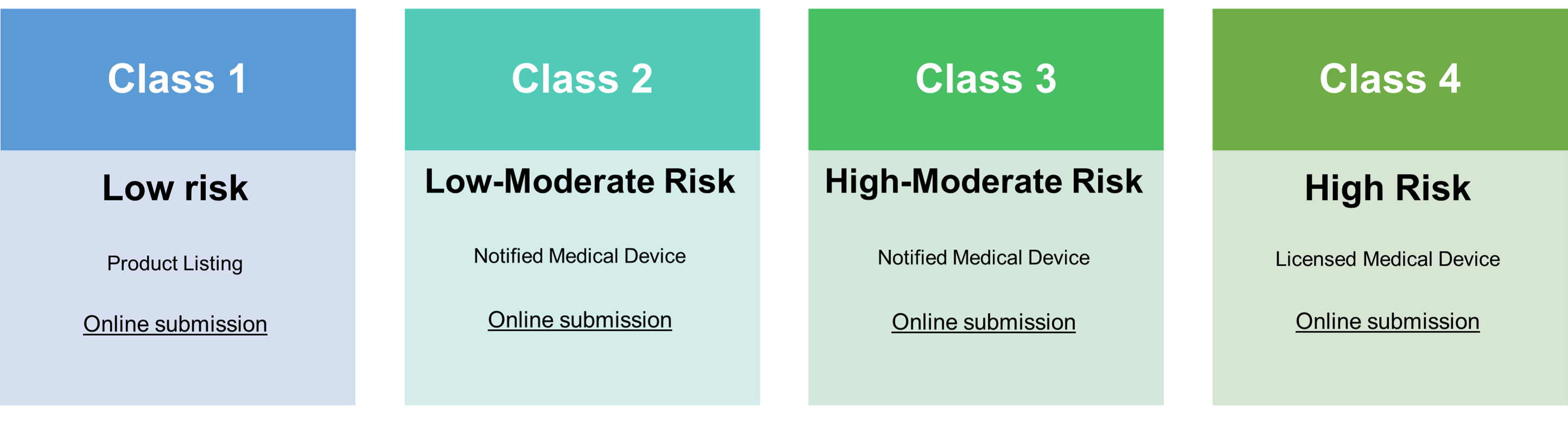
Note: Thai FDA currently follows Risk-based for medical device classification. Therefore, applicants are required to categorize the risk classification. (Please click here for guidance in Thai.)
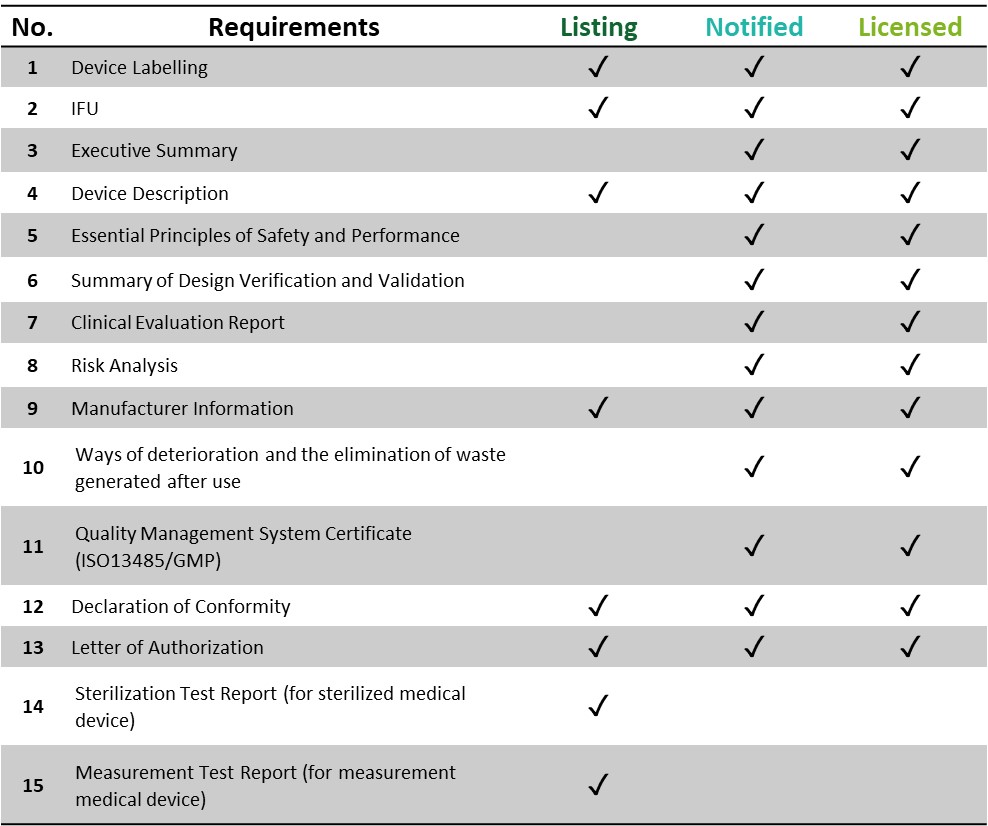 |
|---|
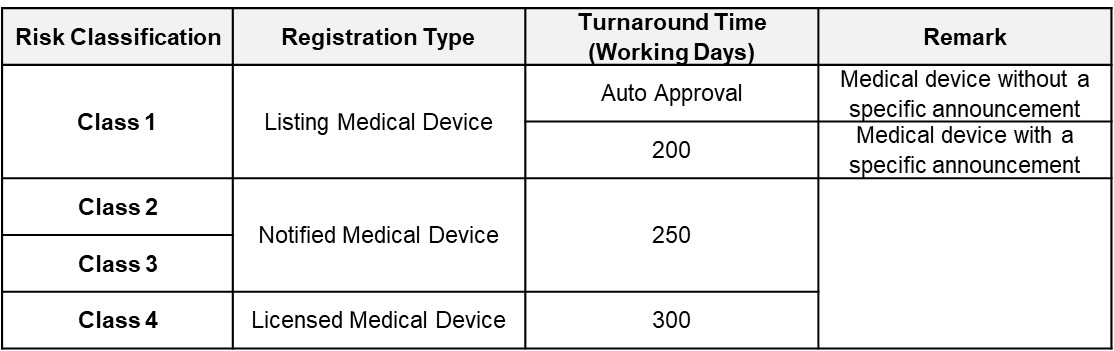
FDA Target Reviewing Evaluation Time
After successful internal evaluation of the submission dossier, our in-house experts will then submit it to the FDA and will be subjected to thorough evaluation. Below is a list of the evaluation times for different types of transactions involving product registration/notification.
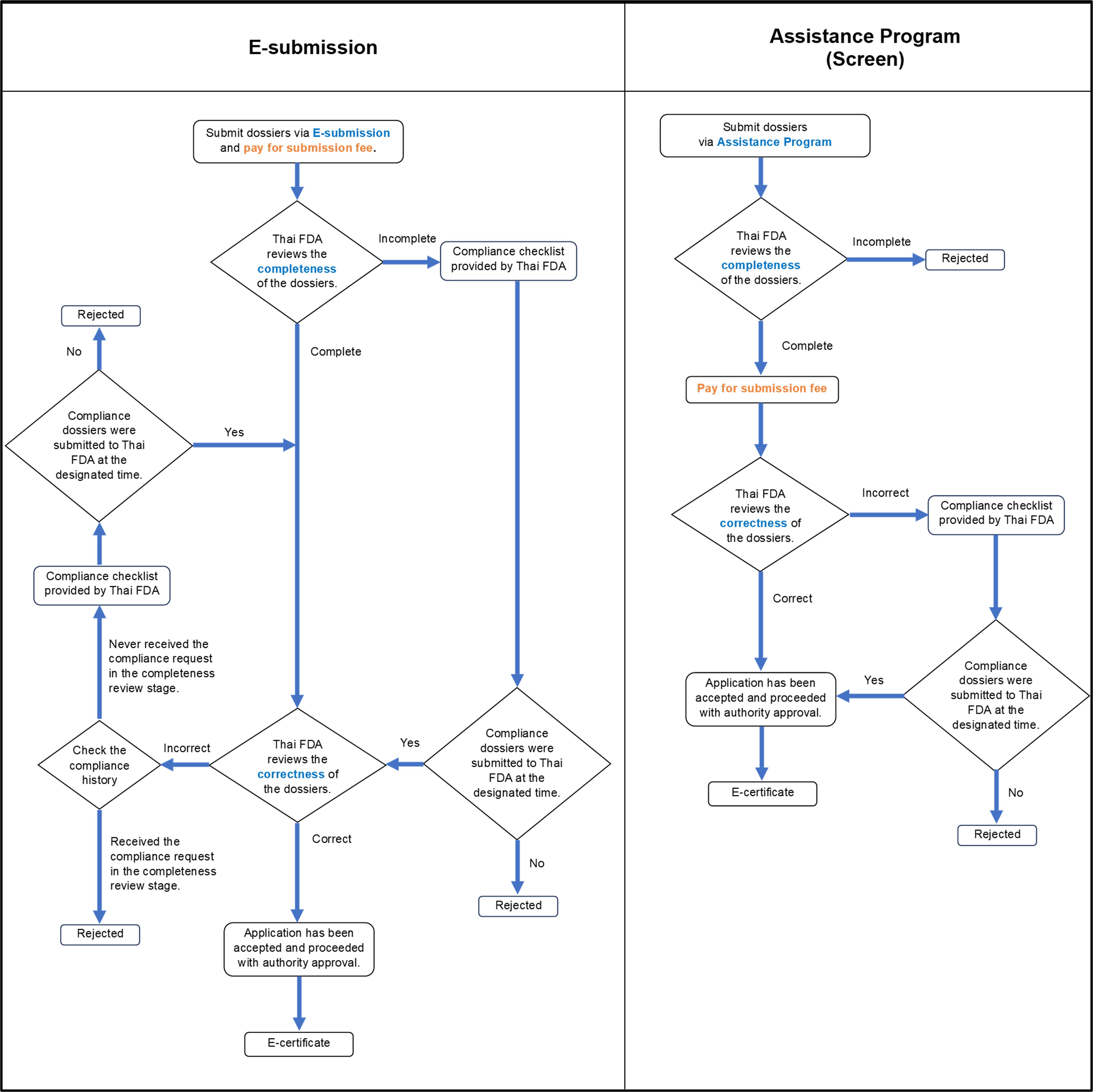 Note: Thai FDA has been consistently working towards improving the overall registration process. As of July 1st, 2023, an updated procedure has been put in place, which allows the screening stage to be utilized as an assistance program. This means that applicants have the option to choose whether to proceed with the initial submission along with the screening stage. This amendment aims to provide greater flexibility and convenience to applicants while maintaining the focus on ensuring the completeness and accuracy of the submitted documents.
Note: Thai FDA has been consistently working towards improving the overall registration process. As of July 1st, 2023, an updated procedure has been put in place, which allows the screening stage to be utilized as an assistance program. This means that applicants have the option to choose whether to proceed with the initial submission along with the screening stage. This amendment aims to provide greater flexibility and convenience to applicants while maintaining the focus on ensuring the completeness and accuracy of the submitted documents.
-
Types of Certificates: Listing, Notified, Licensed
-
Valid for 5 years
-
The applicable expedited route from the Thai FDA supplies much-needed clarity for manufacturers, allowing them to better forecast the potential impact on timelines and costs for certain products. The selection of expedited routes allows applicants to comply according to their medical device approval status.
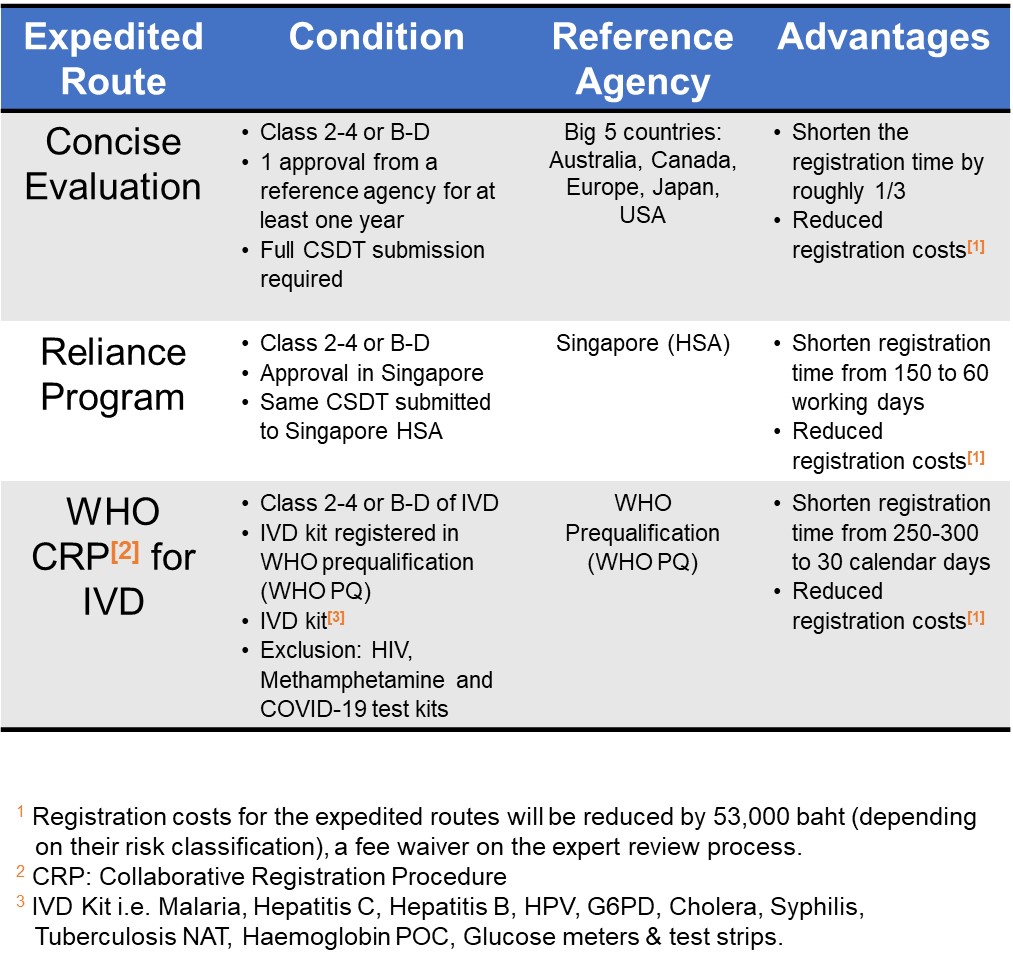
|
The Notice of Importer |
||
|
Importers must make sure that before every importation of medical device, establishment import license part of “Specialty” has covered the product’s UMDNS code. If the specialty category of the Establishment Import License did not cover the UMDNS code of the medical device expected to be import. |
|
|
|
Importer required to secure a total of three Licenses upon proceeding custom clearance:
For more information, please refer to the Thai FDA official website at Thai FDA's official website or you may contact us for a free consultation. |
||
- Thai FDA Medical Device Division
- Thai FDA Import and Export Inspection Division
- Thai FDA Product Database
- Ministry of Public Health Announcement regarding Medical Device Risk Classification 2019
- ThaiFDA Issues the Amended Medical Device Act (Issue 2) B.E. 2562 (2019)
- Thai FDA's Applicable Conditions and Criteria for Expedited Medical Device Registration Routes – May/June 2023
- THAILAND: New Guidance Document on Labelling of Medical Device
Wir verwenden Cookies, um Ihnen ein optimales Website-Erlebnis zu bieten und um die Zugriffe auf unserer Internetseite analysieren zu können. Klicken Sie auf "Ich stimme zu", um Cookies zu akzeptieren und direkt zur Website weiter zu navigieren.
