Australia stands as a beacon of opportunity, with its flourishing medical device sector poised for remarkable growth. Qualtech delves into the market's current dynamics, regulatory landscape, and emerging trends. With in-depth knowledge, Qualtech guides you through documentation and submissions, allowing you to focus on your core business.

Medical Device Registration
Qualtech is your expert for streamlined medical device registration in Australia. Our dedicated team ensures your devices meet The Therapeutic Goods Administration (TGA) regulatory standards seamlessly. From initial consultations to TGA approvals, Qualtech offers comprehensive support, navigating the complexities with efficiency. Rely on our proven success record as we assist you in swiftly and compliantly bringing your medical devices to the Australian market.

Local Sponsor
Qualtech is a qualified sponsor in Australia, we can be your local representative for ensuring compliance with TGA, device importation and post-market surveillance.
Overview of Australian Regulation
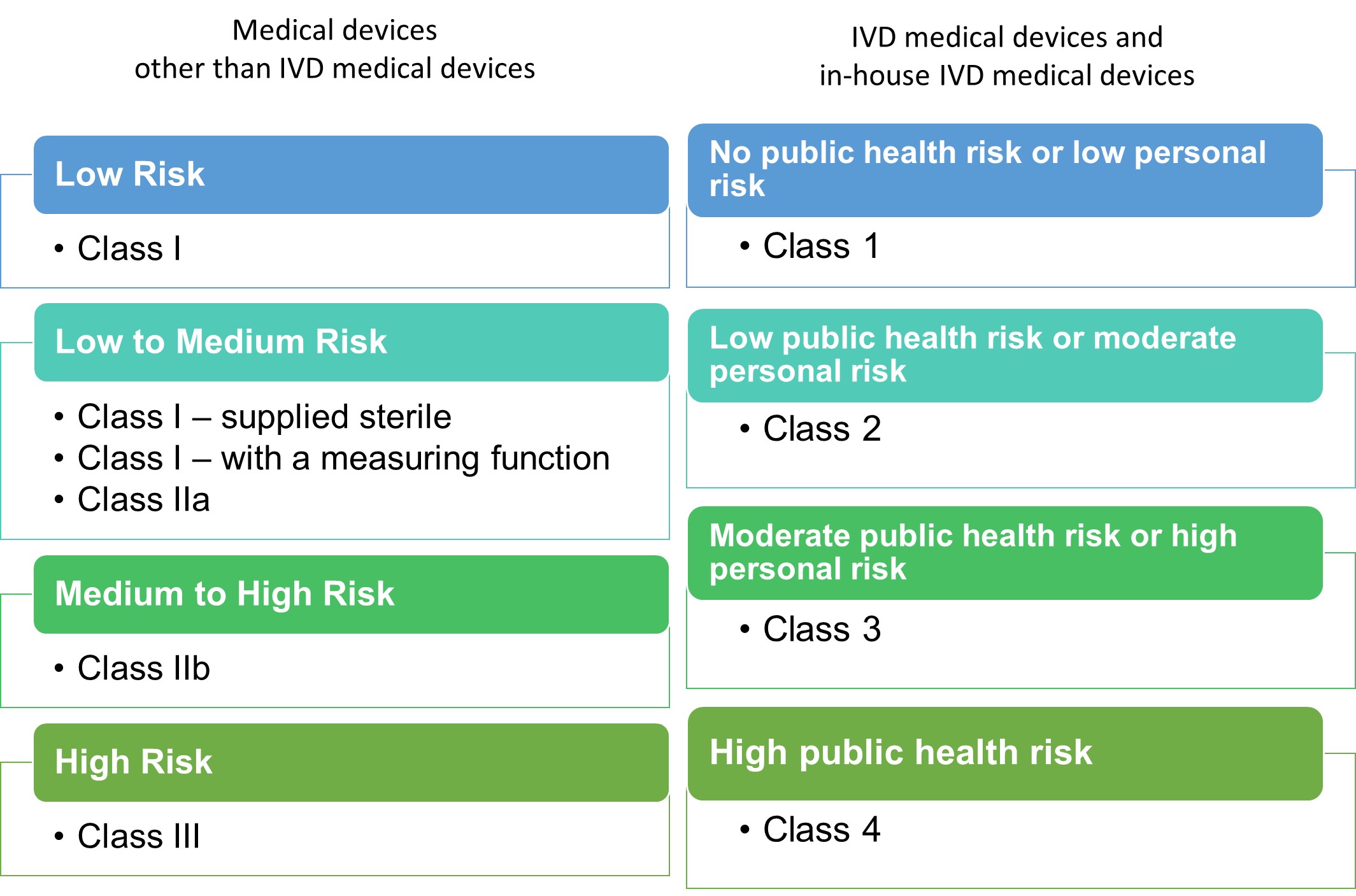
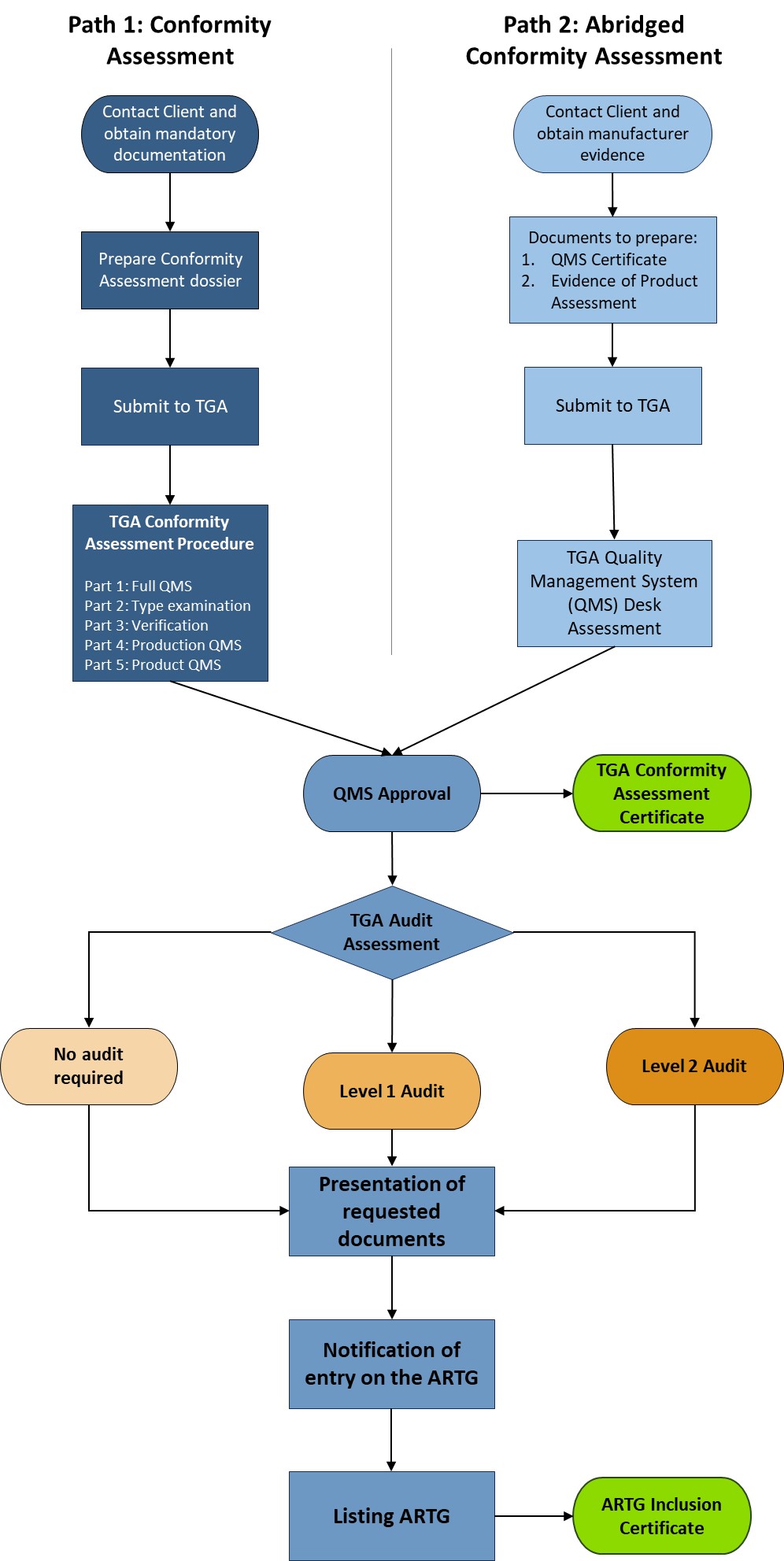
The Therapeutic Goods Administration (TGA) accepts two ways to assess the eligibility of manufacturing facilities for registering imported medical devices in the Australian Register of Therapeutic Goods (ARTG). The first way is TGA Conformity Assessment Certificate, in which TGA evaluates applications based on conformity assessment procedures in Therapeutic Goods (Medical Devices) Regulations 2002. The alternative way is Overseas market authorisation evidence, which entails providing specific evidence and documentation from overseas regulators and assessment bodies. This evidence is reviewed by TGA for potential streamlining of conformity assessments.
Manufacturers must designate a sponsor for regulatory oversight of registered devices. This sponsor must be an Australian resident or an incorporated entity conducting business in Australia, with the company's representative also residing in Australia. The sponsor ensures compliance with therapeutic goods regulations, manages device export, import, or manufacturing, registers therapeutic goods on the ARTG, and oversees post-market surveillance.
TGA Target Reviewing Evaluation Time
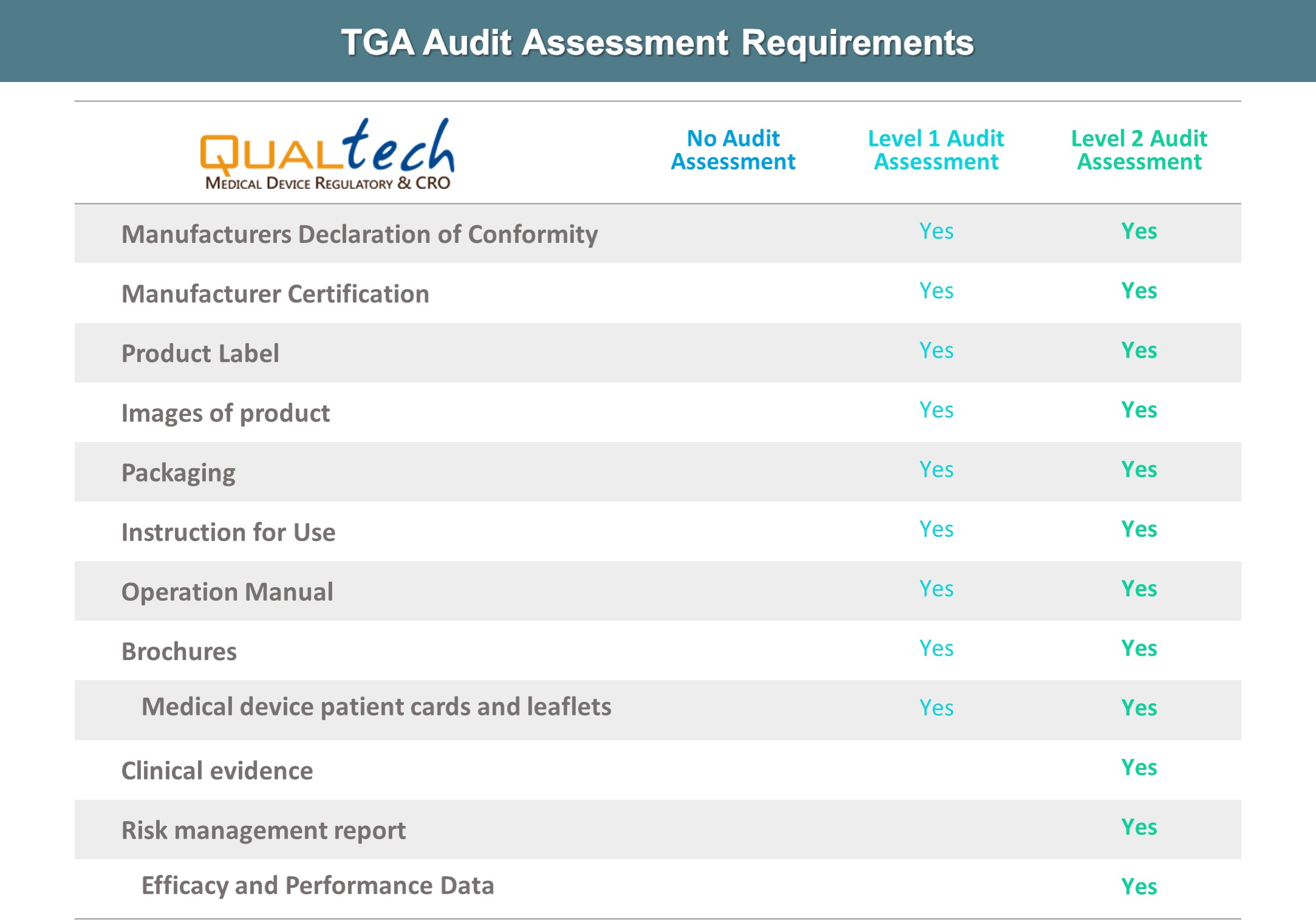
After successful internal evaluation of the submission dossier, our in – house experts will then submit it to the TGA and will be subjected to thorough evaluation. Below is a list of the turnaround times for different types of transactions involving product registration/notification.
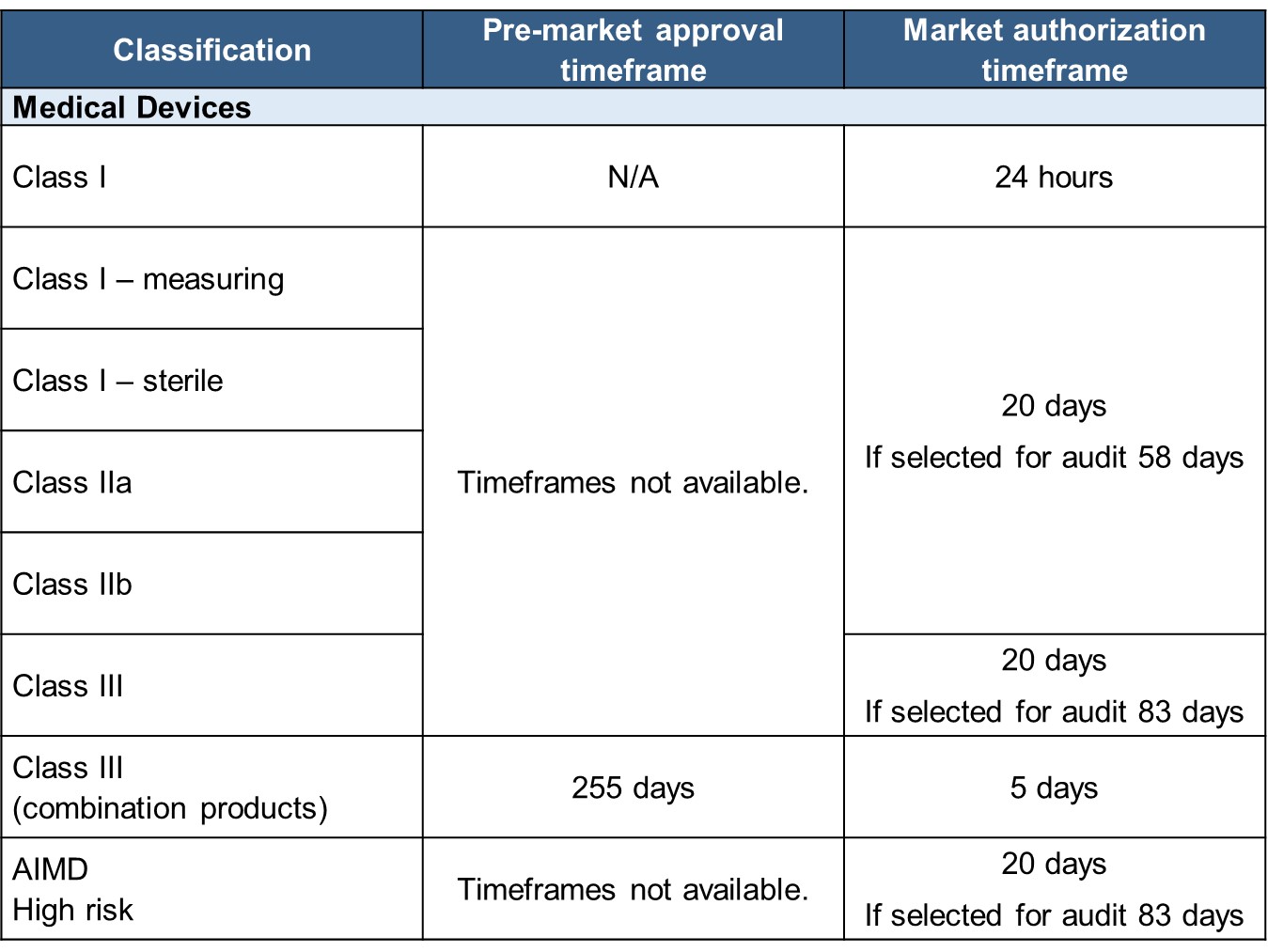
Note: Kindly see the Medical device application processing times more details.
- • TGA Conformity Assessment Certificate: 5 years
- • Manufacturer Evidence: Per overseas certificates' validity.
Manufacturer evidence includes conformity assessment documents within the TGA's quality management system (QMS). The purpose of implementing manufacturer evidence is to ensure the quality of manufacturers before the registration of the ARTG entry.
TGA reviews the overseas market authorisation evidence to potentially simplifying conformity assessments based on the risk classification of the device and the source of that documentation, for:
- • QMS: conformity assessment documentation for a manufacturer's Quality Management System (QMS) issued by issued by other overseas regulators and assessment bodies, that the TGA will accept as manufacturer evidence to support inclusion of medical devices in the ARTG.
- • Product assessment: TGA will acknowledge regulatory approval product certificate as evidence of product assessment to support inclusion of medical devices in the ARTG.
For more information, please refer to the Australia TGA’s official website or you may contact us for a free consultation.
▪ Therapeutic Goods Regulations 1990
▪ Therapeutic Goods (Medical Devices) Regulations 2002
▪ Medical device application processing times
▪ Comparable overseas regulators for medical device applications
▪ AUSTRALIA: USE OF MARKET AUTHORISATION EVIDENCE FROM COMPARABLE OVERSEAS REGULATORS / ASSESSMENT BODIES FOR MEDICAL DEVICES (INCLUDING IVDS)
▪ AUSTRALIA: EFFECTS ON AUSTRALIA REGISTRATIONS BY THE EU MDR TRANSITION
▪ AUSTRALIA: TGA LAUNCHES THE MEDICAL DEVICES VIGILANCE PROGRAM (MDVP) PILOT
Wir verwenden Cookies, um Ihnen ein optimales Website-Erlebnis zu bieten und um die Zugriffe auf unserer Internetseite analysieren zu können. Klicken Sie auf "Ich stimme zu", um Cookies zu akzeptieren und direkt zur Website weiter zu navigieren.
