As one of Singapore's Health Science Authority (HSA) ongoing initiatives to improve regulatory efficiency, adopting a modern regulatory framework that embraces agile methodologies and risk-based assessment is considered necessary. This will help expedite the approval process for certain changes, especially those aimed at maintaining and improving Software as Medical Device (SaMD) effectiveness and safety. HSA first introduced the concept of Change Management Program (CMP) during an industry consultation held from 26 August 2024 to 21 October 2024. This resulted in the pilot voluntary launch of the Change Management Program, which started on 4 December 2024
CMP streamlines the SaMD Total Product Life Cycle-based regulatory framework to facilitate the timely implementation of software changes for SaMD registered on the Singapore Medical Device Register (SMDR). CMP also introduces the concept of pre-specified changes, allowing manufacturers to implement anticipated SaMD changes which would otherwise require a new CN submission, to be implemented in a timely manner.
Along with the launch of the program, HSA also published guidance document GN-37 Change Management Program (CMP) for SaMD, including Machine learning-enabled SaMD. The document outlines the regulatory requirements and procedures for submitting a CMP application. Including the scope of the program, eligibility criteria, application process, pre-specified changes, submission requirements, post-CMP approval, change notification, and turn-around-time also fee of the program.
Scope of the Program
The guidance is applicable to all SaMD, including machine-learning enabled SaMD (ML-SaMD) which are conforming to medical device definition based on Health Products Act 2007. However, this program excludes SaMD implementing continuous learning (CL) and generative AI.
Eligibility Criteria
The Product Owner should comply to the latest standards and possess a valid ISO 13485/MDSAP certificate with approved related scope and IEC 62304 certificate issued by accredited third-party certification body or in-house assessed summary report conforming to IEC 62304. The conformance shall remain valid throughout the SaMD total product life cycle (TPLC).
Application Process
Registrant can enroll into CMP through either an initial Premarket Product Registration or Change Notification. CMP and product registration/CN will be reviewed concurrently, while the regulatory outcome will be determined independently as illustrated below. Additionally, only pre-specified changes which deemed qualified may be approved for implementation.
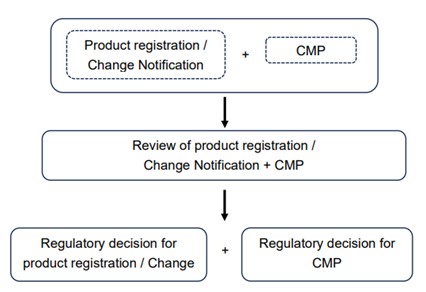
Thus, in case of CMP submission was not approved after review, this will not affect the outcome of initial product registration or change notification.
Pre-specified Changes
Pre-specified changes shall be submitted along with the CMP application. Pre-specified changes shall include specific changes that Product Owner intends to implement, that would otherwise require a new CN application. The Pre-specified changes shall fall within the intended use and original indication for use of the product. Such changes only include those that are intended to maintain or improve the safety and effectiveness of the product without introducing new safety risks.
The published guidance GN-37 outlined the examples of changes that may qualified to be submitted under CMP, also the ones that do not qualify to be submitted. Changes that do not qualify to be submitted under CMP such as change in intended use and/or indication for use of the original device, change in method of use, changes to device particulars which are published on SMDR, also changes related to FSCA and/or reportable AE. Kindly refer to the guidance document for the detailed explanation.
Submission Requirements
The published guidance provided the list and explanation on the required submission documents, whether it is submitted during initial product registration or Change Notification application. Kindly refer to Section 5. Submission Requirements of GN-37, additionally the summary of the submission requirements also available in Annex I of the document.
Post-CMP Approval
Approved Pre-specified changes under CMP may be implemented without Change Notification submission. A Declaration on the implementation records should be submitted within 1 year after approval of CMP application. The next declaration shall be submitted within 1 year from the last declaration submission.
Product Owner should ensure that appropriate mechanisms are in place to differentiate and identify the changed SaMD, maintain relevant inventory records to ensure traceability of the versions. All relevant records on file shall be made available to the Authority upon request.
Turn-Around-Time (TAT) and Fees
There’s no additional/difference in TAT and Fees for CMP enrolment. The time and fee will follow the respective Product Registration or Change Notification type which as available in HSA website.
Change Notification
Change Notification (CN) should be submitted if there’s any changes to the previously submitted pre-specified changes. In the published GN-37, HSA outlined the detailed guiding principles on how to determine the category of change notification should be selected.
Call-to-Action
Navigating through innovatively dynamic regulatory environment in Singapore can be challenging. However, the pilot-run CMP program offers valuable support for managing the rapid iterative nature of your SaMD product and expediting the implementation of updates.
Qualtech Consulting Corporation has been a trusted partner for medical device manufacturers for over 20 years. Whether you’re a local startup or an international player, we empower your devices to enhance lives.
Connect with us today to unlock your medical device potential.
References
- Pilot Run: Change Management Program (CMP) for SaMD, including Machine Learning-Enabled SaMD
- Guidance on Change Management Program (CMP) for SaMD, including Machine Learning-Enabled SaMD (GN-37)
