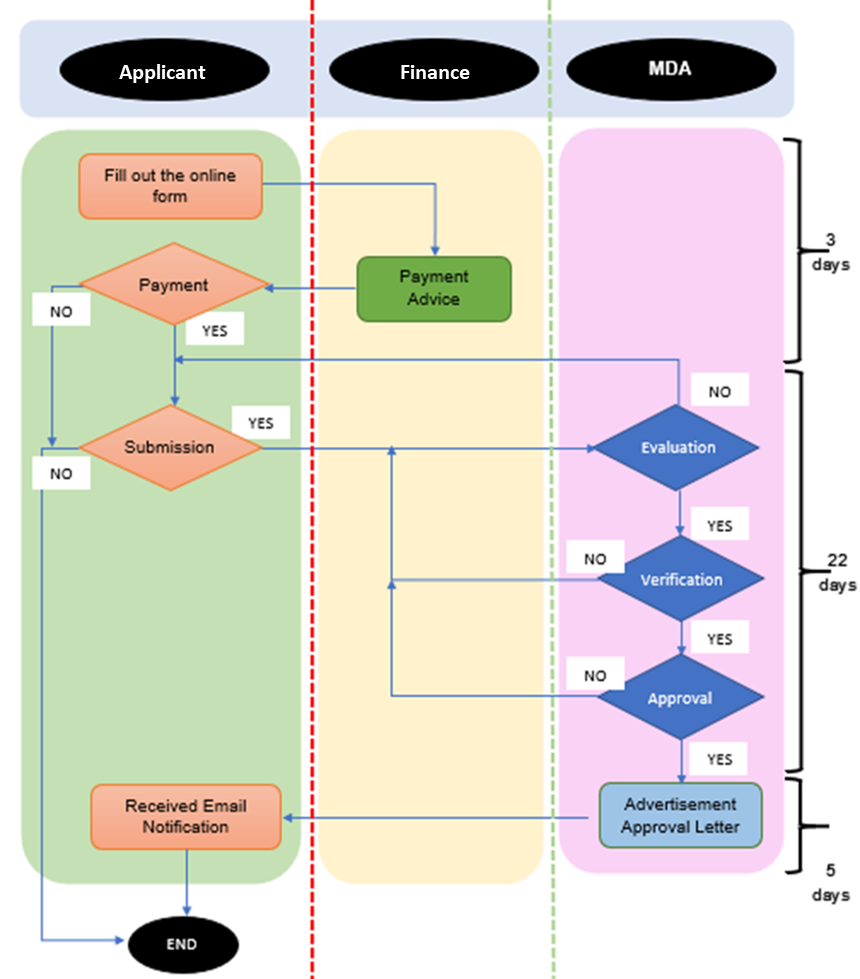
As of 1st March 2025, the Medical Device Authority (MDA) officially transitioned the medical device advertisement application process to a fully online format via Google Form. This change was implemented to improve efficiency, simplify submissions, and enhance the overall experience for applicants.
Key Updates:
- Online Submission Implemented: All medical device advertisement applications are now required to be submitted exclusively through the designated Google Form.
- Email Submissions Discontinued: Applications submitted via email are no longer accepted. Incomplete applications are rejected, and applicants must re-apply using the online form.
- Digital Notification Process: Approved advertisement letters are now sent directly to the applicant's email address. No physical copies are issued or mailed.
Stakeholder Advisory:
MDA urged all stakeholders to:
- Familiarize themselves with the online submission procedure.
- Ensure that all required documents and information are complete before submitting.
- Re-submit via the online form if previous applications were sent via email
References:
Official Announcements:
- MDA Main Page: Medical Device Advertisement Process
- Official Announcement: Transition to Online Advertisement Application
Guidance Documents:
- MDA/GD/0032 – Code of Advertisement
- Guidelines: Application for Medical Device Advertisement Approval – Requirements
- Advertisement Application Form (Physical)
- Advertisement Application Online Form
- Template: Advertisement Letter of Authorisation
