HSA’s Medical Devices Cluster has released a draft of Guidance on Change Management Program (CMP) for stakeholders with consultation period from 26 August 2024 to 21 October 2024. Change Management Program (CMP) is a new optional regulatory pathway for SaMD, this new pathway is incorporated into HSA’s Premarket Product Registration and Change Notification (CN) process. The aim of this framework to accommodate the rapid iterative nature of SaMD changes. The CMP also introduces the concept of Pre-specified changes, this concept is allowing upcoming anticipated changes for the SaMD to be implemented in a timely manner.
CMP streamlines SaMD Total Product Life Cycle-based (TPLC-based) regulatory framework to facilitate timely implementation of software changes for SaMD and to reduce redundancy in dossier submission. This is done by establishing confidence in the Product Owner’s good quality management system practices, demonstrated through excellent capabilities in their SaMD development, verification/validation, and post-market surveillance/vigilance.
In addition, with a pre-existing device approval under CMP, this program facilitates leveraging on previously approved CMP documentation if the subsequent device registration is for a similar SaMD with equivalent quality management processes.
The DRAFT of Guidance for CMP contains explanation on:
1) Eligibility Criteria to enroll into CMP,
2) Application Process,
3) Submission Requirements,
4) Post-CMP Approval,
5) Change Notification,
6) Leveraging on Approved CMP, and
7) Turn-around-Time (TAT) and Fees.
While the scope applicable to all SaMD, including machine-learning (ML) incorporated SaMD (ML-SaMD) with intended use that falls under the definition of a medical device.
Eligibility Criteria
The Product Owner should comply to the latest standards and possess a valid ISO 13485 certificate with approved related scope and IEC 62304 certificate issued by accredited third-party certification body or in-house assessed summary report to IEC 62304, etc.
Application Process
Registrant can enroll into CMP through either a Premarket Product Registration or Change Notification (Review Change (Class B) or Technical Change (Class C)). CMP and product registration/CN will be reviewed concurrently, while the regulatory outcome will be determined independently as illustrated below:
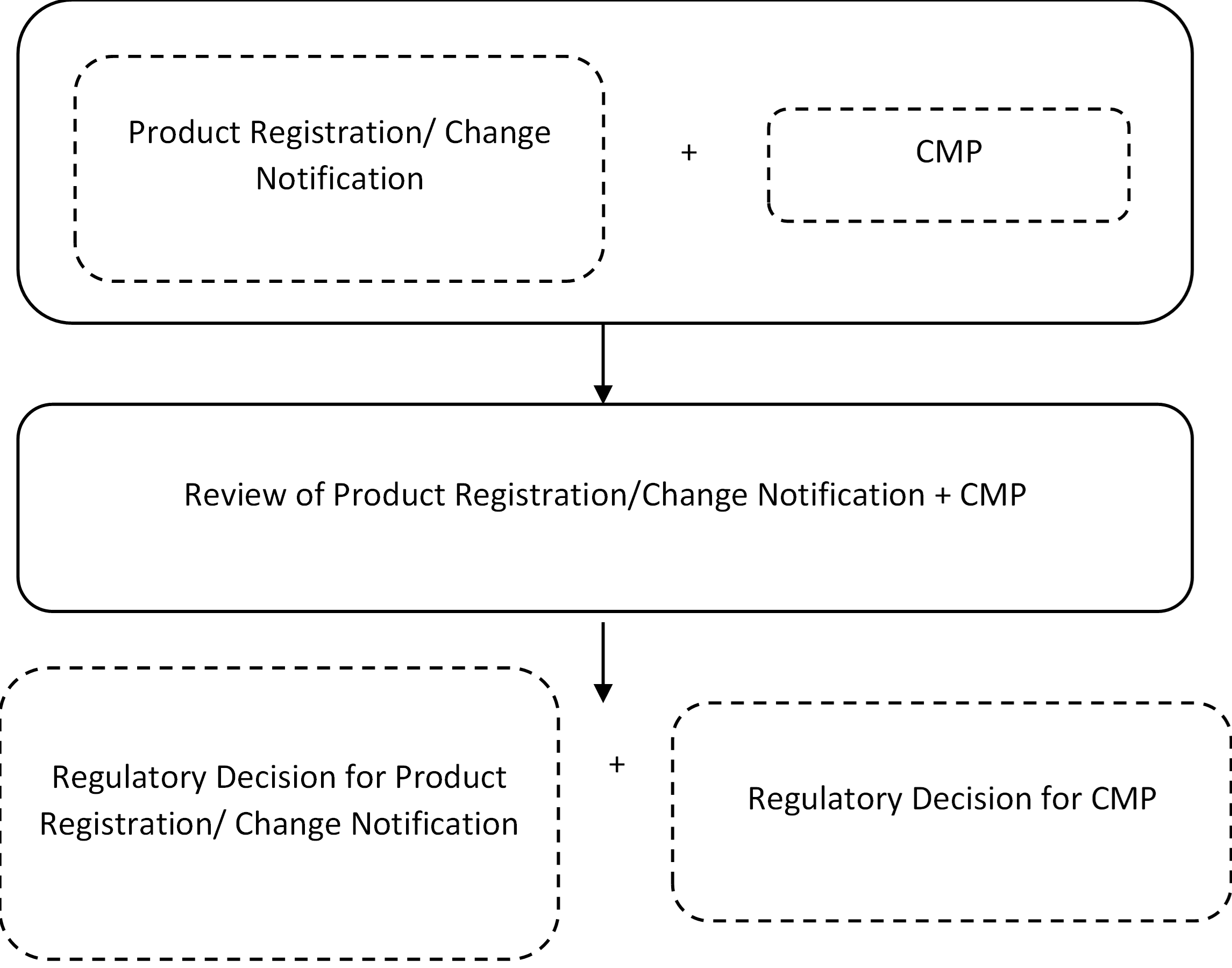
Submission Requirements
Documents demonstrating good quality management system practices through excellent capabilities in SaMD development, verification/validation, post-market surveillance/vigilance to ensure the safety, effectiveness, and cybersecurity of the SaMD throughout their TPLC. Product Owner shall provide justification or alternative information, as applicable, if any of the following outlined information is not available.
Referring to the Annex 2 template as provided in the draft GN, Quality Assurance Processes should demonstrate following:
- Timely review of recognized standards throughout SaMD TPLC, timely review or gap analysis to assess whether SaMD conforms to the latest applicable standard.
- SaMD versioning and traceability processes, to ensure traceability during post-market by providing SaMD software versioning explanation and 3rd party and/open-source software record-keeping and monitoring process.
- Cybersecurity and data safety management, by providing documents showing IEC 61001-5-1 conformance where applicable, or full set of cybersecurity requirements.
- Safety issues management, including effective Adverse Events (AE) and Field Safety Correction Action (FSCA reporting)
- Additional process related to Risk Management for SaMD with third-party and open-source software throughout the SaMD TPLC.
- Post Market data analysis
Pre-specified Changes
Applicable for SaMD with upcoming anticipated-changes (e.g: improvement in existing features/specifications, bug fixes, etc) that would otherwise require a new Change Notification application, except changes resulting in change in intended use, indication for use, and method of use, also changes to device particulars which are published on SMDR.
Requirements:
- Change Description
- Implementation protocol, describing how the changes will be implemented and managed.
- Performance verification and validation protocol, describing the process that will be followed to demonstrate that the changed SaMD will meet the new specifications as well as maintain the existing specifications.
- For ML-SaMD (except continuous learning (CL) and generative AI): i) Training test dataset selection/collection, ii) Re-training protocol.
- Traceability table (for multiple changes only) related to relevant protocols.
- Post-implementation impact analysis, containing the risks and benefits analysis of implementing the pre-specified changes, as well as the mitigations of the identified risks.
Post CMP Approval
Approved Pre-specified changes under CMP may be implemented without Change Notification Submission. Registrant shall be required to submit a Declaration on the implementation records within 1 year after approval of CMP application. The next Declaration on the implementation records shall be submitted within 1 year from the last declaration submission.
Product Owner should ensure that appropriate mechanisms are in place to differentiate and identify the changed SaMD, maintain relevant inventory records to ensure traceability of the versions. All relevant records on file shall be made available to the Authority upon request.
Change Notification
Under following circumstances, Change Notification should be applied instead:
|
Type of Changes |
Categories of Change |
Submission Requirement |
|
Change to previously approved Pre-specified changes |
Notification change |
|
|
Addition of new Pre-specified changes |
|
|
|
All other changes (not described above) |
Refer to GN-21: Guidance on Change Notification For ML-SaMD, refer to Regulatory Guidelines for Software medical Devices – A Life Cycle Approach |
|
Notification changes may be implemented immediately upon receipt of the acknowledgement email from HSA after submission via MEDICS. Changes under Notification change type may be bundled and notified to HSA in one change notification application. Alternatively, such changes could be submitted together with the next Review/Technical change. While bundling Notification changes, any such change shall be submitted within a maximum of 6 months from the point of first implementation, globally. Relevant inventory records on file should be maintained to ensure traceability. Bundled Notification Changes do not apply to AI based devices and AE/FSCA related changes.
Leveraging on Approved CMP
To reduce redundancy in CMP dossier preparation and facilitate faster market access for similar SaMD, Product Owner may leverage on the approved CMP documentation in new Product Registration on similar SaMD or CN for registered SaMD (Review Change for Class B, Technical change for Class C).
Requirements:
- Evidence of conformity to ISO 13485 & IEC 62304
- Justification for SaMD used as reference in the previously approved CMP submissions remains applicable to the new SaMD to be registered/already-listed SaMD
- Pre-specified changes documents, if applicable in case different from the pre-specified changes authorized for the previously approved SaMD under CMP
- Declaration letter from Product Owner stating that the quality management process for the new SaMD/already-listed SaMD are equivalent to the one reviewed previously as reference.
- Reference CMP-approved SaMD device name and SMDR listing number.
Turn-around-Time (TAT) and Fees
There’s no additional/difference in TAT and Fees for CMP enrolment. The time and fee will follow the respective Product Registration or Change Notification type which as available in HSA website.
Call-to-Action
The Consultation period for the draft Guidance on Change Management Program (CM) for SaMD is from 26 August 2024 to 21 October 2024. Feedback should be emailed to HSA using the prescribed feedback form to HSA_MD_INFO@hsa.gov.sg by 21 October 2024 and with "Feedback on Guidance on CMP for SaMD" in the email subject header.
Qualtech Consulting Corporation has been a trusted partner for medical device manufacturers for over 20 years. Whether you’re a local startup or an international player, we empower your devices to enhance lives.
Connect with us today here to unlock your medical device potential.
References:
