NMPA of China released the registration work report in April 2020, covering the milestones of medical device regulations and registration applications from last year. In addition to actively improving relevant regulatory regulations and standards, NMPA also gradually adjusts its internal operating methods, such as developing online submission systems, establishing clinical departments, and optimizing more efficient review systems.
I Medical Device Regulation:
- Regulation System Overview
(1) Promote the Revision of Medical Device Regulations.
Cooperate with the Ministry of Justice to improve the regulations, and simultaneously update the “Regulation of Medical Device Distribution” and “Regulation of Adverse Event for Medica Device”.
(2) Improve the Medical Device Standard System.
As of the end of 2019, there are 1,671 current effective standards in the field of medical devices, including 220 national(GB) standards and 1451 industry(YY) standards. The consistency of our standards with international standards has reached 90.4%.
(3) Promote the Formulation of Guidelines for Medical Device
57 guidelines of medical device were implemented, including the "Guidance for Clinical Evaluation of Medical Devices", to uniform and improve the review capacity of each branch FDA.
2.Reform of Medical Device Registration System
(1) Optimize Clinical Trial Approval
The "Announcement on Adjusting the Approval Procedures for Clinical Trials of Medical Devices" (Announcement No.26 of 2019) was issued to further optimize the clinical trial approval procedures, and the "explicit" license was adjusted to the "implicit" license.
(2) Expand the List of Medical Devices Exempt from Clinical Trials.
Published the "Revised Lists of Medical Devices Exempted from Clinical Trials" (Announcement No. 91 of 2019). Currently, the list of medical devices exempted from clinical trials contains 1003 items, and IVDs list contains 416 items A total of 1419 items were reached.
(3) Accelerate the Approval of Innovative Medical Devices.
In 2019, a total of 36 innovative products and 12 priority approval products entered the "Green channel". Approved 19 innovative medical products such as positron emission tomography and magnetic resonance imaging systems, and 10 priority products such as hollow fiber membrane hemodiafiltration filter.
(4) Expand the Pilot of the Medical Device Registrant System.
The “Notice of the NMPA on Expanding the Pilot Work of the Medical Device Registrant System” was issued, this “Notice” is to cover the pilot of the medical device registrant system to 21 provinces, including Beijing, Jiangsu and Zhejiang. In 2019, a total of 93 products from 22 companies were approved under the registrant system pilot, including cross-provincial commissioned production and Class III medical devices through the registrant system commissioned production and other different situations.
3. Digital Supervision on Medical Devices.
(1) Advance e-Submission system
Publish the "Announcement on the Implementation of E-submission of Medical Device Registration" and establish an online system (also named as eRPS). In 2019, 7512 applications were submitted via this new system.
(2) Optimize the Reviewing Workflow
Achieve graded review of simple cases and complex cases, for example, initial registration, major changes application, and clinical trial approval are taken as complex cases, which are handled by newly established project management group. In addition, CMDE has established an independent department for clinical evaluation and statistics.
(3) Implement the Unique Device Identification (UDI) of Medical Devices
Publishing "Medical Device Unique Identification System Rules" and related standards. NMPA clarified the variety scope, schedule and work requirements of trial program, in addition, several manufacturers join this program. The trial program will be completed in June 2020.
II Acceptance Performance of Registration Application
In 2019, there were 9,104 applications submitted to NMPA, including initial registration, renewal, and changes applications with an increase of 37.8% compared to 2018. Finally 5593 of which were accepted and turn around to the reviewing stage.
- Registration Acceptance of Imported Class II Devices
Last year, 3053 registrations of Class II imported device were accepted, an increase of 43.7% compared with 2018. Among them, 1559 applications are for medical device and 1494 projects for in vitro diagnostic reagent registration.
From the perspective of registration form, 358 items were initial application; 1847 items were renewal application; 848 projects were major change application. The distribution of registration forms is shown in Fig.1.
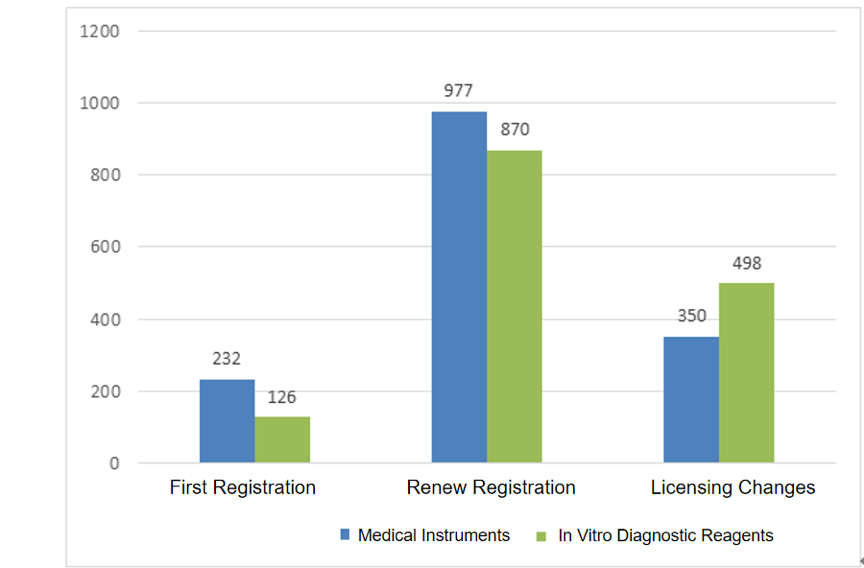
Fig.1 Distribution of the accepted application of imported Class II product
- Registration Acceptance of Imported Class III Devices
Number of Imported Class III device application is 2540 items, an increase of 20.9% compared with 2018. Among them, there were 2164 medical device applications and 376 for in vitro diagnostic product.
From the perspective of registration form, 280 items were initial application; 1457 items were renewal application; 803 items were major change application. The distribution of registration forms is shown in Fig.2.
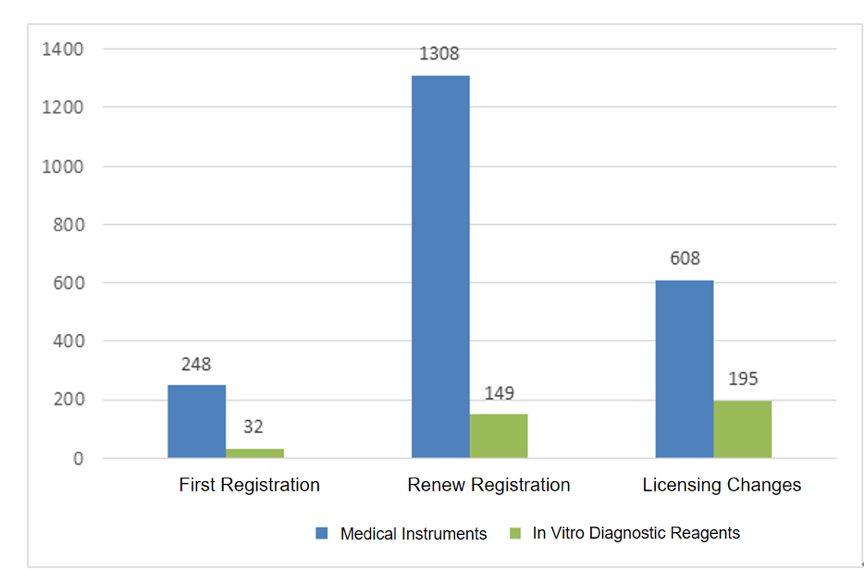
Fig.2 Distribution of the accepted application of the imported Class III device
III Approval Performance of Registration Application
- Approval Performance of Imported Medical Devices
(1) Imported Class II Medical Devices
Imported 2754 items of Class II medical devices. Among them, there were 1521 projects are medical device and 1233 projects are in vitro diagnostic device.
From the perspective of registration form, 375 items were initial application; 1,622 items were renewal application; 757 items were major change application. The distribution of approval performance is shown in Figure 3.
