In mid-May 2019, India’s central medical device regulator, the Central Drug Standards Control Organization (CDSCO) has issued a risk classification of all newly notified medical devices and IVDs on the basis of their intended use, under the provisions of the Medical Device Rules 2017. Before this, CDSCO had issued plans of including more types of medical devices as being regulated, ranging from all implantable medical devices, CT, MRI & X-ray scanning equipment, dialysis machines, to nebulizers.
According to the new regulations, general medical devices of these newly notified sets will be classified on the basis of parameters specified in Part I of the First Schedule, namely low risk as class A; low to moderate risk as Class B; moderate to high risk as class C and high risk as Class D.
IVD medical devices will be classified on the basis of parameters specified in Part II of the First Schedule, namely low risk as Class A; low to moderate risk as Class B; moderate to high risk as Class C and high risk as Class D.
The latest notification issued by CDSCO in this regard reads:
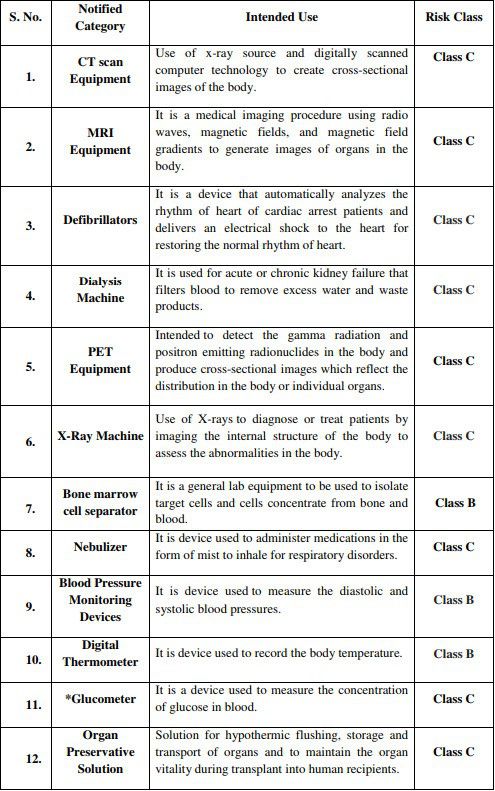
* Glucometer will be regulated under IVD category
It is to be noted that prior to this, CDSCO has already notified 383 general medical devices and 247 IVD devices as regulated medical devices in India, according to Medical Device Rules 2017. Along with these new additions, the number now stands at 394 general medical devices and 248 IVD medical devices, having accorded their own risk classification and will be fully implemented starting April 1, 2020. Kindly refer to the official Notice from CDSCO in the reference section below, to peruse the full list.
References:
Classification of newly notified Medical Devices (29/Misc/3/2017-DC (292))
