Understanding the Health Sector of the Philippines - The Rise of Lifestyle Diseases:
Based on the information collected by the Statistics Bureau of the Philippines, trends clearly show that Lifestyle Diseases such as the Ischemic Heart Disease, Diabetes, and Obesity have been on a dramatic rise in the Philippines. Tabaco; for instance, is quite affordable in the country, which is believed to have significantly influenced the results obtained. In response, the Philippine President Rodrigo Duterte issued a nationwide smoking ban in 2017, which subsequently prohibited smoking in public places. Shortly afterwards in January 2018, the Philippine government furthermore introduced a tax applying to all sweet beverages. Hence, the Philippine government is strongly signaling its efforts in containing the spread of such lifestyle diseases.
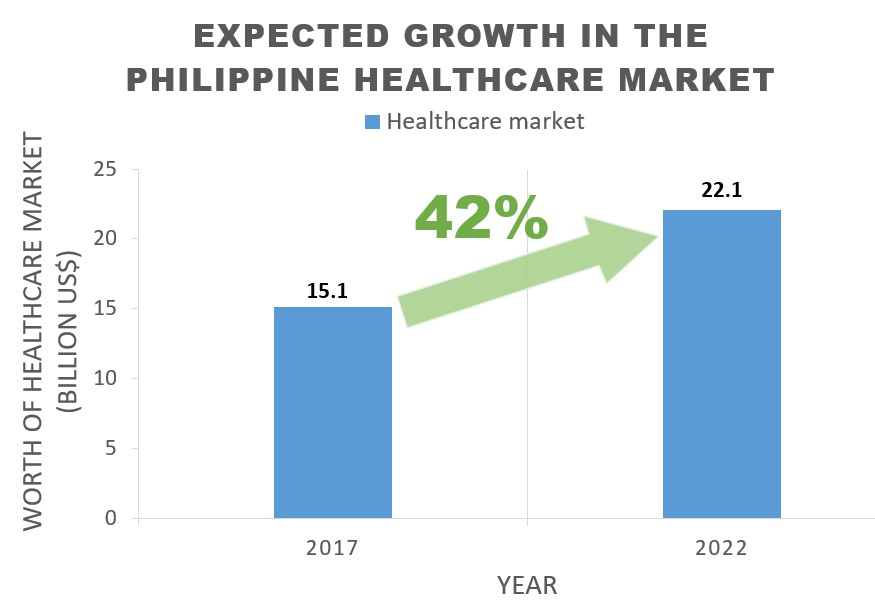
Apart from that, the Philippine’s Social Security System (hereinafter referred to as ”SSS”) is well constructed and includes a pension system as well as National Health Insurance. The SSS is providing services to all people of the Philippines, even including those who work in foreign countries. According to Philhealth’s statistical data, 93% of the Philippine population is currently being covered by the National Health Insurance, and a further increase is being predicted. Simultaneously, the healthcare market itself is also expected to significantly grow from 15.1 billion USD in 2017 to approx. 22.1 billion USD by 2022.
Source: Business Monitor International (2017)
Favorable Conditions for Foreign Medical Device Manufacturers in the Philippines:
Although Generic Drugs dominate, the size of the medical device market is nevertheless quite substantial with 332 million USD in 2018. Moreover, the medical device market is expected to achieve a growth rate of over 10%. Factors that contribute to this strong growth are the frequent renewal of medical equipment and the experienced rise in medical tourism. Surprisingly however, 90% of the medical devices in use in the Philippines are being imported. Products from the United States had by far the largest market share in the year 2018. US products were then followed by medical devices from Germany, Japan, and Singapore (each one; however, only roughly amounting to half of the number of US products). In fact, the Philippines has not significantly developed its own manufacturing industry of medical devices yet. According to the latest data consulted, there are currently less than 30 entities concerning medical device manufacturing in business in the Philippines.
Local production thereby primarily focuses on the creation of prototype units, and disposables, including surgical gloves, syringes, and needles. Particularly due to this fact, the Philippine Medical Device Market offers a large amount of opportunities for foreign manufacturers, as local competition is not as strong as in other Asian markets. When looking at the main medical device importers of the Philippines above, one also notices that big global medical device producer countries - such as Japan, Germany and other European countries - are momentarily not holding a prominent share of the market.
Conditioned by the increasing demand for medical device equipment - caused by a growing request for innovative devices from abroad, hospital expansions, and a steady rise in population and economic growth - favorable market conditions for foreign medical device manufacturers can be met in the Philippines.
How to Enter the Market of the Philippines:
In order to leverage from these beneficial conditions, Qualtech therefore offers a broad amount of services via our local team of regulatory affairs specialists in the nation’s capital, Manilla. Apart from efficiently guiding clients through the product registration process, Qualtech also offers additional services to comply with other related rules and local regulations. We can thus serve as a License Holder and Local Authorized Representative for interested manufacturers. A local registrant, who holds an LTO (License to Operate), is required in the Philippines when obtaining a Certificate of Product Registration (CPR) or a Certificate of Exemption (CoE) for non-registrable devices.
As importation can occasionally represent a rather tricky subject, Qualtech may also further assist in ensuring that products pass smoothly through customs clearance. If desired, Qualtech may provide additional support with local distribution. Ultimately aiming at providing a comprehensive and one-stop solution for your activities in the Philippines.
The Philippine Medical Device Regulations in Times of Change:
When playing with the idea of bringing a medical device to the Philippine market, manufacturers have to take note that the Philippines are currently in a transition period regarding their medical device regulations. That is, as the Philippines are moving towards harmonization with the rest of the ASEAN region under the ASEAN Medical Device Directive (AMDD). Under the current regulations, which are still valid at this point, the Philippines are following a registrable/non-registrable classification system. In the future, registration will follow the CSDT format (Common Submission Dossier Template) for class B, C, and D devices according to the new risk-based classification. Implementation will be conducted in 3 stages later this year with a starting date that still has to be announced by the PFDA. Readers interested in the upcoming changes and the core elements of the revised regulations may directly contact Qualtech for free consultation. Alternatively, we have also prepared detailed articles on the Registration in the Philippines (General) and the 6 Expected Big Changes in Philippine Medical Device Registration for your further information.
References:
- JETRO (Japan External Trade Organization)
- EU Gateway – Business Avenues
