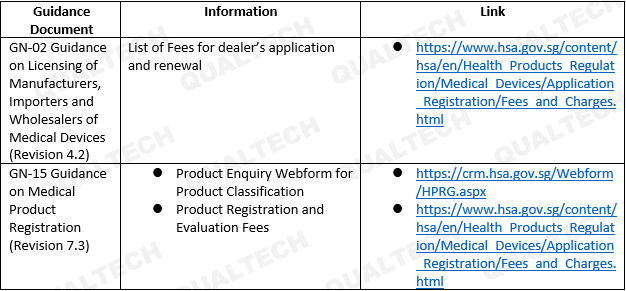
The latest revision for both guidance documents adds important links to salient information in the guidance. Please refer to Table 1 for more details.
References:
Reference:
GN-02: Guidance on Licensing of Manufacturers, Importers and Wholesalers of Medical Devices
GN-15: Guidance on Medical Product Registration