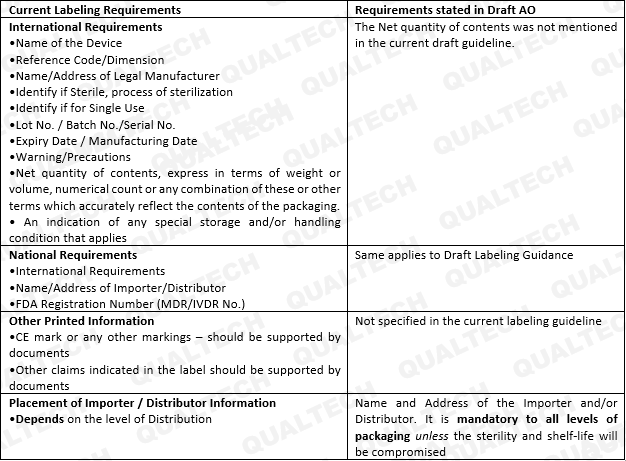
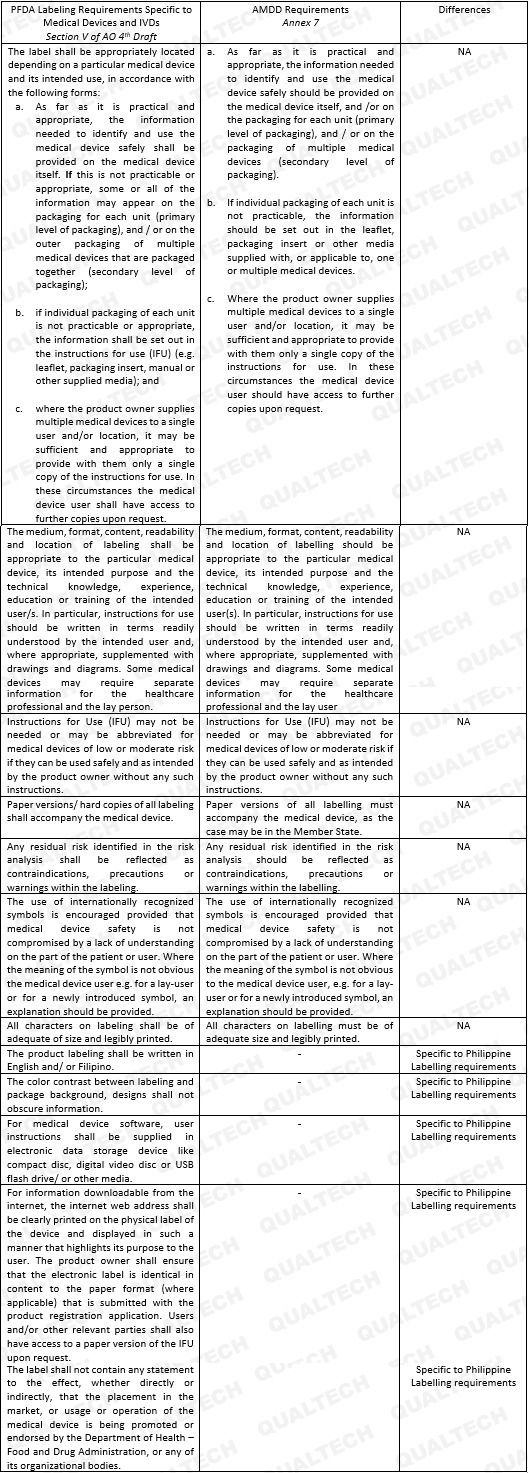
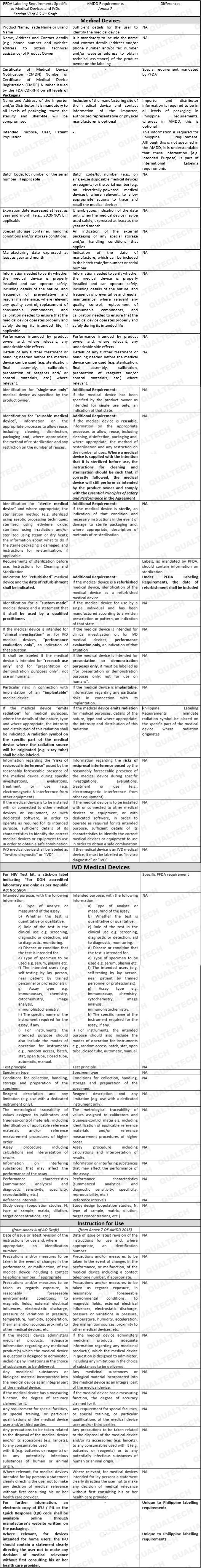
Recently, the Philippine FDA has published a newly drafted guidance for Labeling. The new guidance was catered to effectively communicate safety instructions to end-users and patients alike, as well as to standardize labeling requirements well aligned with the labeling provisions set in the AMDD. This guidance applies to all medical devices, including in-vitro diagnostics (IVD) currently being marketed in the Philippines.
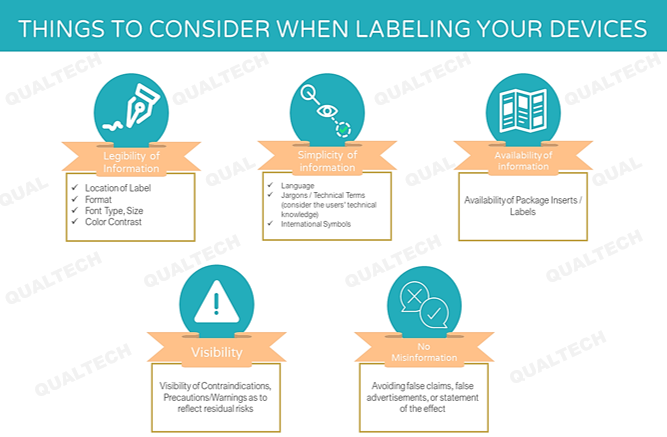
The general provisions concern itself on the legibility of salient information related to device use (e.g., where the label shall be placed, font type and size, color contrast, format), comprehensibility of information commensurate to the level of understanding of the end-users/patient (e.g., medium of instruction, jargons, use of internationally-recognized symbols), provision of labels/package insert in paper version, visibility of contraindications, precautions/warnings as to reflect residual risks, and misinformation due to false claims. All general information shall be located depending on a particular medical device and its intended use.
References:
ASEAN Medical Device Directive
Draft of New Philippine Labeling Requirements