In May 2018, MDA had issued a Circular Letter (No.4 Y 2018), and enforced the policy exempting export-only medical devices from registration requirement. Since these devices will not be placed in the Malaysian market, hence it was deemed not necessary to go through the hassle of registering in Malaysia. However, MDA has made it compulsory for all export-only medical devices manufactured locally to be notified with the agency, during each exportation.
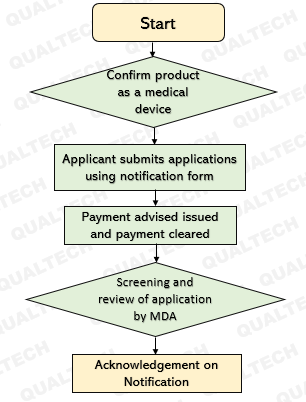
Following the enforcement of this policy, MDA has issued a draft for a guidance document (MDA/GD/0051) for export-only medical devices and their notification with the regulatory body. With this guidance document, steps to be taken to notify MDA on the export-only medical devices, have been clearly specified. When a manufacturer or Authorised Representative notifies MDA about an export-only medical device and its impending exportation, MDA will review the documentation submitted and if everything is agreeable, will issue an “Acknowledgment on Notification” letter, which permits the device to be exported. One notification application shall be made for only one single medical device or one medical device grouping (family of systems is not allowed). Also, one notification application may be submitted for up to 10 countries and 10 brand names (for the same product) from one manufacturer/contract manufacturer.
The notification shall be made according to the Flowchart in Annex A of this Guidance Document.
The list of documents to be submitted along with the application form have been described in detail in Table 1 of this Guidance Document. The applicant shall submit application form by email to exportonly@mda.gov.my. It is expected that the applicant would submit a notification before exportation of the first shipment, so an “Acknowledgement on Notification” will be issued before exportation of the medical device, upon successful application. MDA will process the application as soon as receiving it and expects applicant to provide additional information, particulars or documents within 14 days from the date of request by authority.
Reference: