The Singapore HSA has made a few minor changes to the guideline GN – 21 R4.6: Guidance on Change Notification and was published last February 2020 under the name GN – 21 R4.7. The revisions included are as follows:
- Added “Bundling Together of Notification Changes in One Application” in the Notification Changes part under Categories of Changes section.
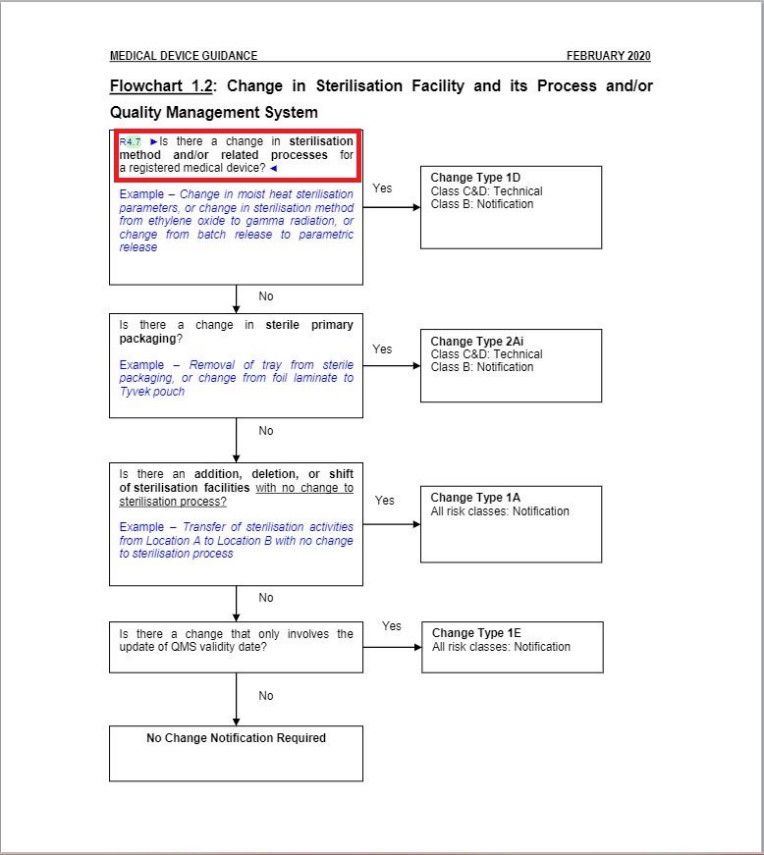
- Revised a part of “Flowchart 1.2: Change in Sterilization Facility and its Process and/or Quality Management System” under Change Type Assessment Flowcharts section.
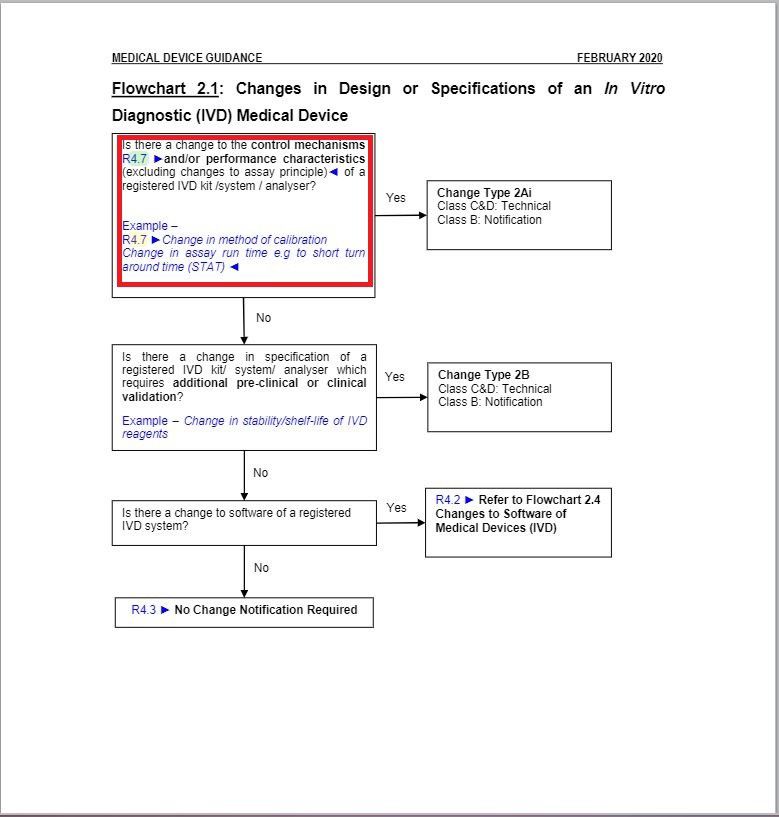
- Revised a part of “Flowchart 2.1: Changes in Design or Specifications of an In Vitro Diagnostic (IVD) Medical Device” under Change Type Assessment Flowcharts section.
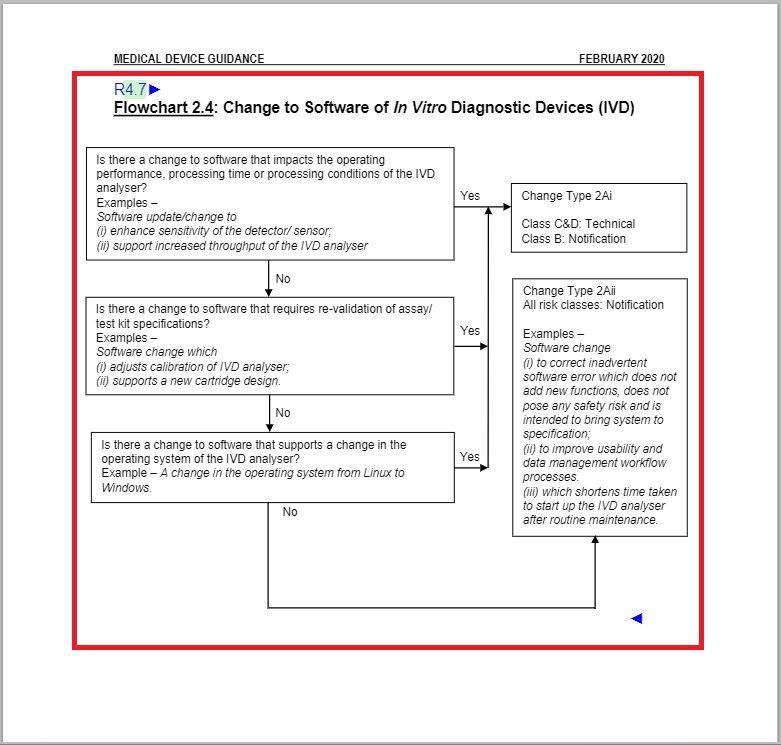
- Revised “Flowchart 2.4: Change to Software of In Vitro Diagnostic Devices (IVD)” under Change Type Assessment Flowcharts section.
- Added “Bundled Notification Changes” under Application Process for Change Notification section.
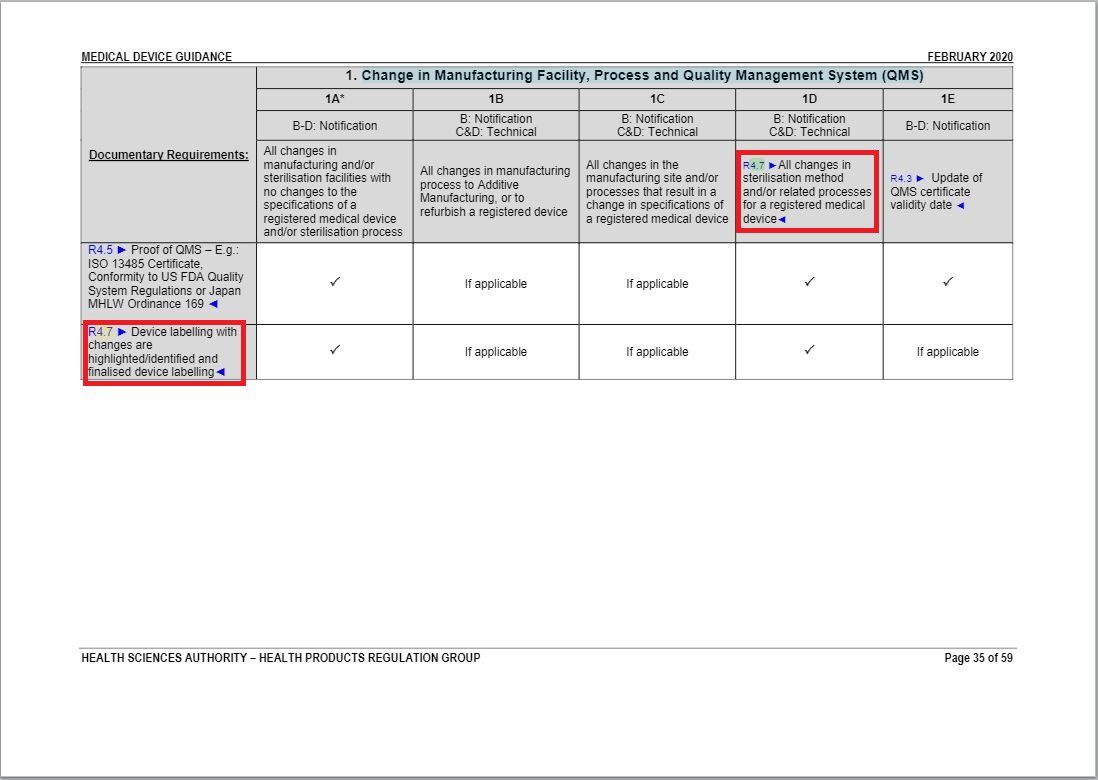
- Revised some parts of “Change in Manufacturing Facility, Process and Quality Management System (QMS) table” underAnnex 1 to GN – 21: Change Notification Submission Requirements.
- Revised a part of “Changes in Design or Specifications of a Registered Medical Device (GMD and IVD) table” under Annex 1 to GN – 21: Change Notification Submission Requirements.
- Added an item in the “Documentation Guidelines for Software Changes table” under Annex 1 to GN – 21: Change Notification Submission Requirements.
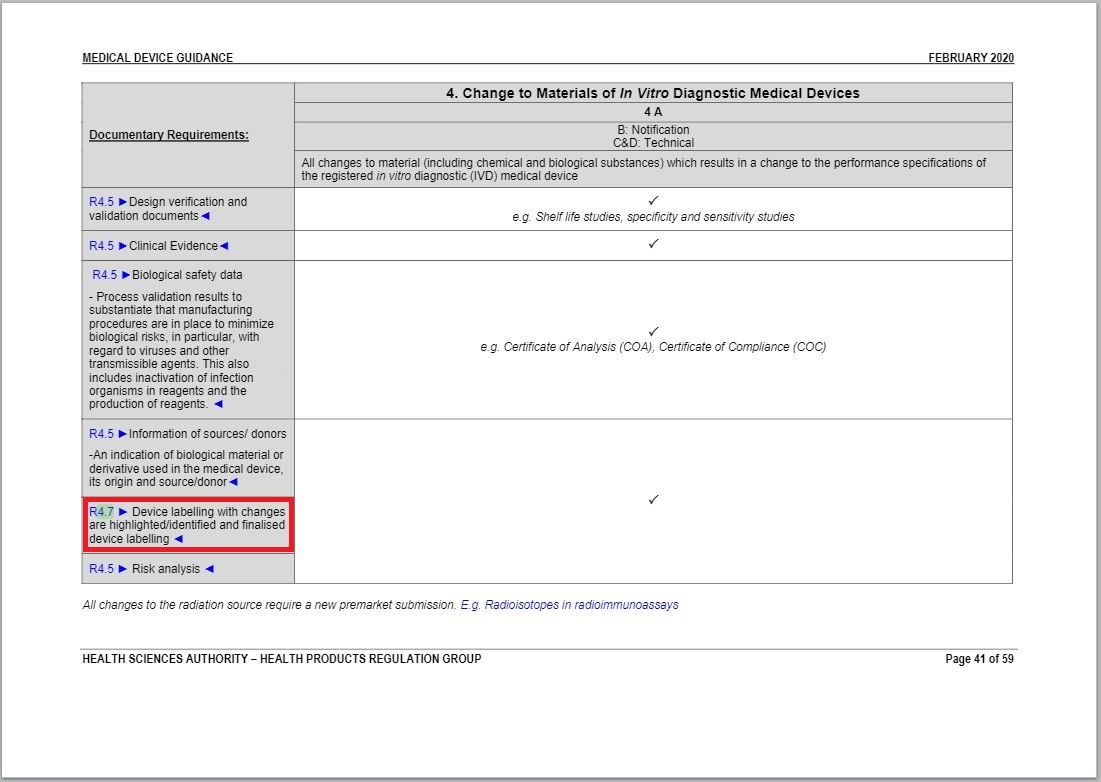
- Revised a part of “Change to Materials of In Vitro Diagnostic Medical Devices table” under Annex 1 to GN – 21: Change Notification Submission Requirements.
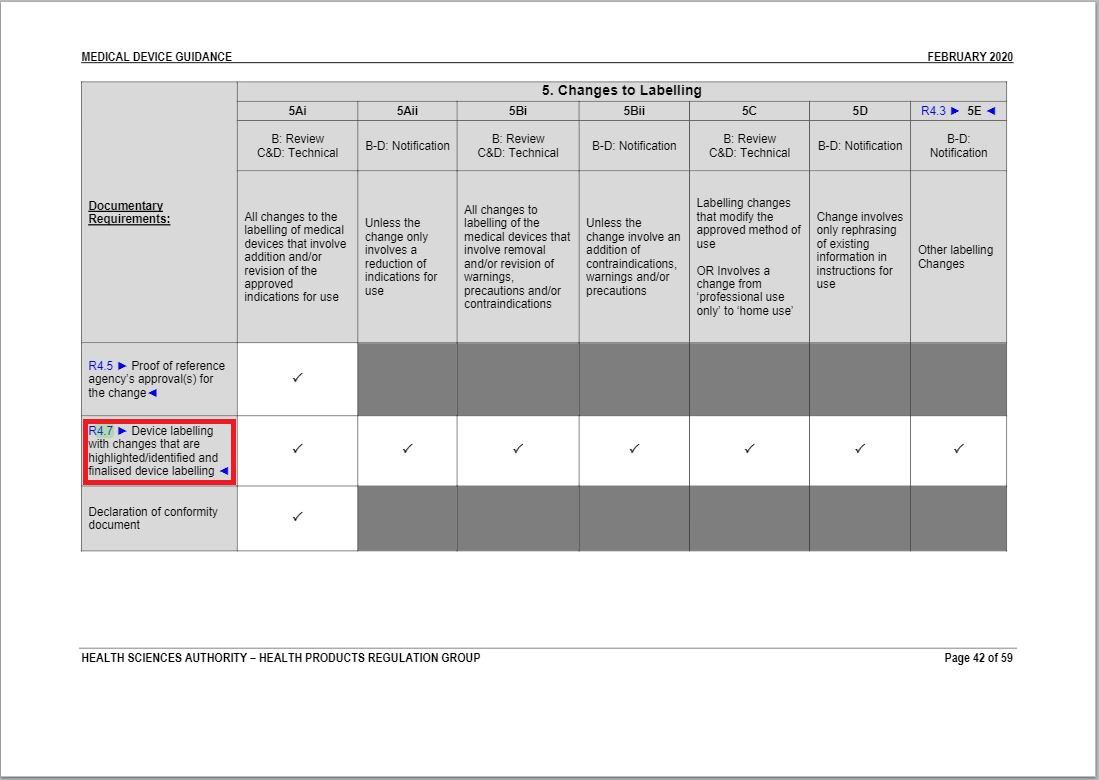
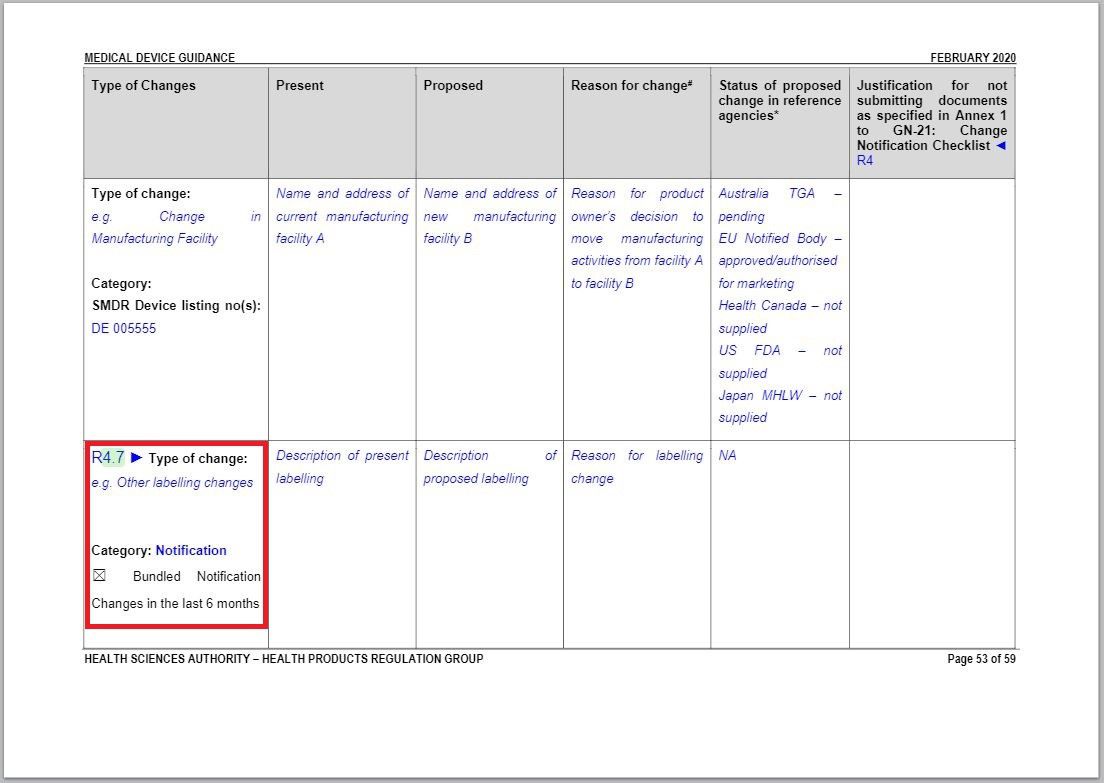
- Revised a part of “Changes to Labelling table” under Annex 1 to GN – 21: Change Notification Submission Requirements.
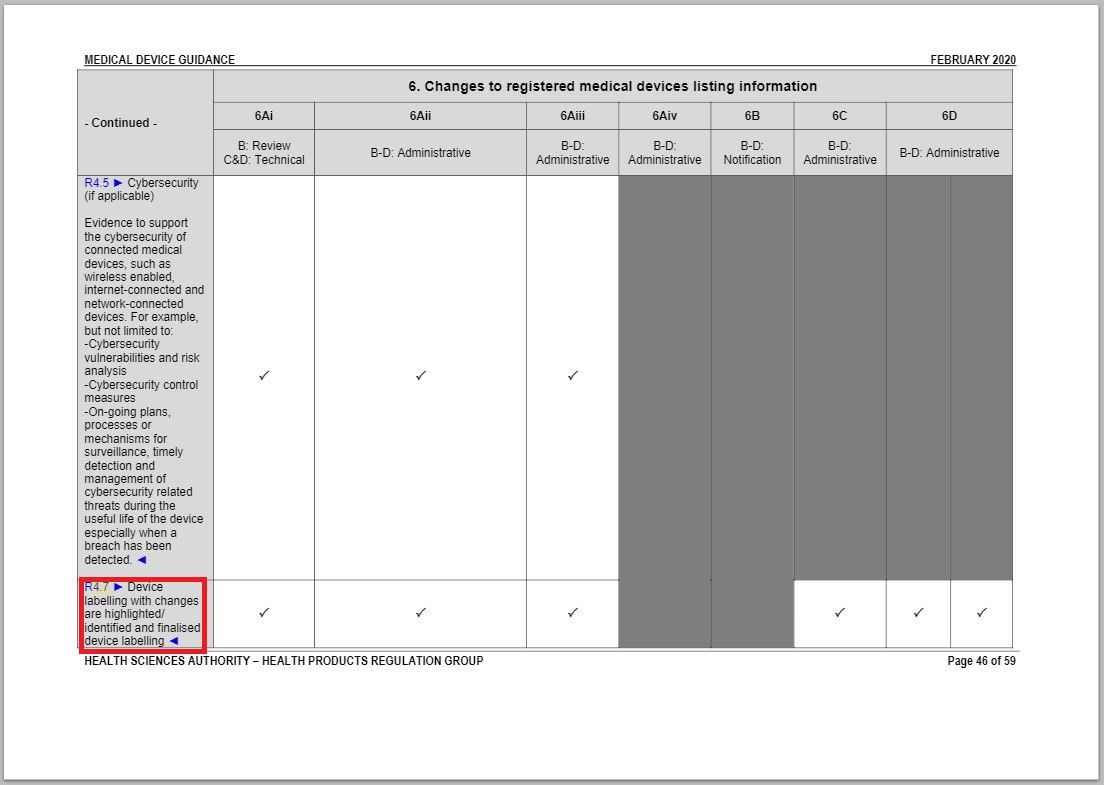
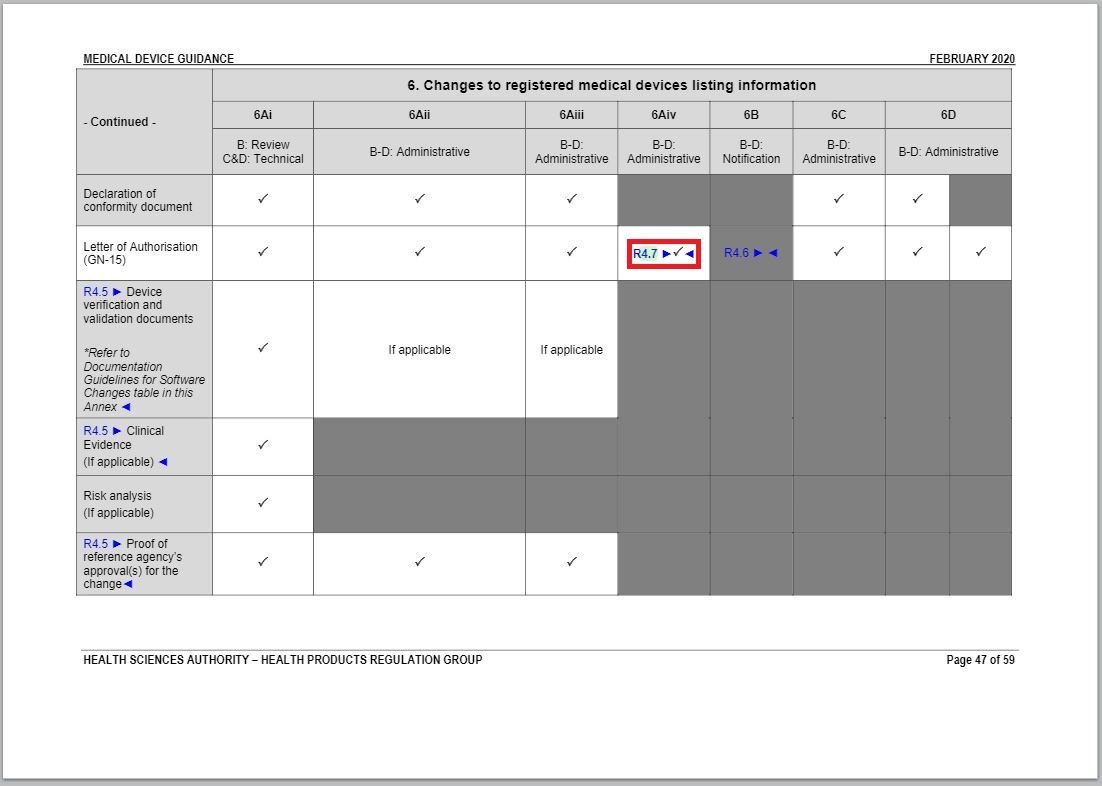
- Added an item to and revised a part of “Changes to Registered Medical Devices Listing Information” under Annex 1 to GN – 21: Change Notification Submission Requirements.
- Revised a part of “Annex 2 to GN – 21: Summary Table of Change Notification”.
The details of the listed revisions of the guidelines can also be found in the following table:
Clarification through Visuals:
Table 1: GN – 21 R4.7 document screenshots showing the details of each revisions.
|
Item |
Remarks |
|
Added “Bundling Together of Notification Changes in One Application” |
Added a new paragraph under the Notification Changes
|
|
Revision in Flowcharts 1.2, 2.1 and 2.4 |
Minor changes in the wordings in Flowchart 1.2
|
|
Minor changes in the wordings in Flowchart 2.1
|
|
|
Revised the entirety of Flowchart 2.4
|
|
|
Added “Bundled Notification Changes” comment |
Minor addition regarding Bundled Notification Changes
|
|
Revision in Change in Manufacturing Facility, Process and Quality Management System (QMS) table
|
Minor revisions in accordance with Flowchart 1.2
|
|
Revision in Documentation Guidelines for Software Changes table
|
Added some minor notes.
|
|
Revision in Change to Materials of In Vitro Diagnostic Medical Devices table
|
Same with one of revisions of Change in Manufacturing Facility, Process and Quality Management System (QMS) table
|
|
Revision in Changes to Labelling table |
Same with the revision in Change to Materials of In Vitro Diagnostic Medical Devices table
|
|
Revision in Changes to registered medical devices listing information table
|
Same with the revision in Changes to Labelling table
|
|
|
Added a check mark in the cell coinciding Letter of Authorisation row and 6Aiv column.
|
|
Revision in ANNEX 2 to GN – 21: Summary Table of Change Notification
|
Minor addition in Type of Changes column.
|
References:
Reference: GN-21-R4.7 Guidance on Change Notification for Registered MD(Feb-pub)