The Medical Device Rules 2017 has recently been amended and it will now apply to all medical devices, effective April 1, 2020. Prior to the Medical Devices (Amendment) Rules, 2020 was issued, only 37 categories of medical devices were regulated or were notified to be regulated in near future in India.
Sequence of timeline for implementation of the Medical Devices (Amendment) Rules, 2020:
- Before October 1, 2021, all presently unregulated medical devices will have to be registered by respective importers or manufacturers with the Drugs Controller General of India. However, those medical devices which are already regulated or have been notified to be regulated are exempted from the requirement of registration (see list of 37 categories of medical devices at the end of this article which are exempt from registration).
- Before August 11, 2022, importers, manufacturers, distributors, whole sellers and retailers of presently unregulated Class A (low-risk) and Class B (medium-risk) medical devices sold in India will have to compulsorily obtain a license.
- Before August 11, 2023, importers and manufacturers, distributors, whole sellers and retailers of presently unregulated Class C (low-risk) and Class D (medium-risk) medical devices sold in India will have to compulsorily obtain a license.
In order to obtain registration for medical devices, the importers and manufacturers of the medical devices have to be certified as compliant with ISO-13485 (Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes).
The government has given time to the medical device industry to transition into the regulatory framework and to obtain ISO 13485 certification, if not already obtained. The government has relaxed the requirement to obtain registration and license for newly notified medical devices for the following period:
- April 1, 2020 to October 1, 2021 – No registration or license will be required to manufacture, import, distribute or sell Newly Notified Medical Devices;
- October 1, 2021 to August 10, 2022 – Registration will be required to import or manufacture such medical devices, but no license will be required;
- August 11, 2022 to August 10, 2023 – License will be required to manufacture, import, distribute or sell Class A or Class B medical devices, but no license will be required to manufacture, import, distribute or sell Class C or Class D medical devices; and
- After August 11, 2023 – License will be required to manufacture, import, distribute or sell Class C and Class D medical devices as well.
The Amendment Rules also prescribes the information to be uploaded by the manufacturer or the importer of medical device, on the “Online System for Medical Devices” (“Online System”) established by CDSCO, for the registration of the medical devices.
After the required information are uploaded on the Online System, for the registration of medical devices, a registration number will be generated for the applicant. The manufacturer or the importer of the medical devices will be required to mention the registration number on the label of the medical device.
The registration process is relatively simpler and should not be equated to a full-fledged marketing registration or authorization. If an importer or manufacturer is unable to obtain registration for its Newly Notified Medical Device before October 1, 2021, then it will not be able to market and sell its medical device in India until a registration is obtained.
There are 37 categories of medical devices which if regulated or notified before the date of MDR Amendment i.e. February 11, 2020, will not be affected by the MDR Amendment and therefore will not be required to obtain registration. This list of exempted 37 categories of medical devices is:
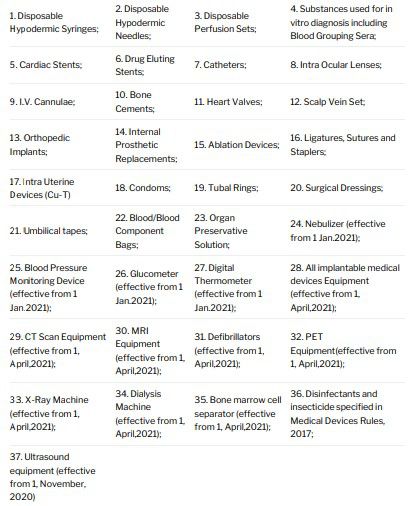
However, being exempted from application of the MDR Amendment does not mean that they are exempted from MDR itself. These devices and their importers, manufacturers and the entire supply chain will have to obtain a license and observe other compliances stipulated under MDR at all times.
In addition to registration, importers and manufacturers of Newly Notified Medical Devices (not notified until February 11, 2020 and will now be covered by new definition of medical device) will have to obtain a license under MDR before the prescribed deadline (see table for deadlines). In the table below, we have listed the name of the authority who will issue the license to importers and manufacturers along with prescribed deadlines.
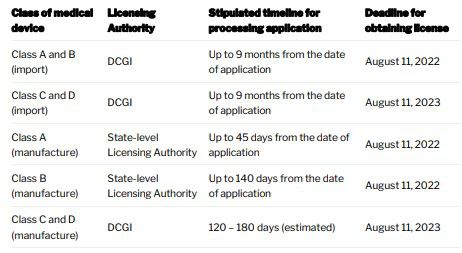
It is important to note that it is not mandatory to have a registration number in order to obtain a license. Therefore, the application for license can be made any time after April 1, 2020 (or such other date that DCGI may specify in future).
The Amendment Rules will empower the Authority to verify the documents relating to the medical devices at any point of time and investigate any quality or safety related failure or complaints. The Authority will also have the power to cancel or suspend for such period of time as it deems fit the registration number of the registrant, either wholly or in respect of any of the medical devices to which it relates, if it is of the opinion that the registrant has failed to comply with any provision of the Principal Rules.
References:
New Medical Devices (Amendment) Rules, 2020
