|
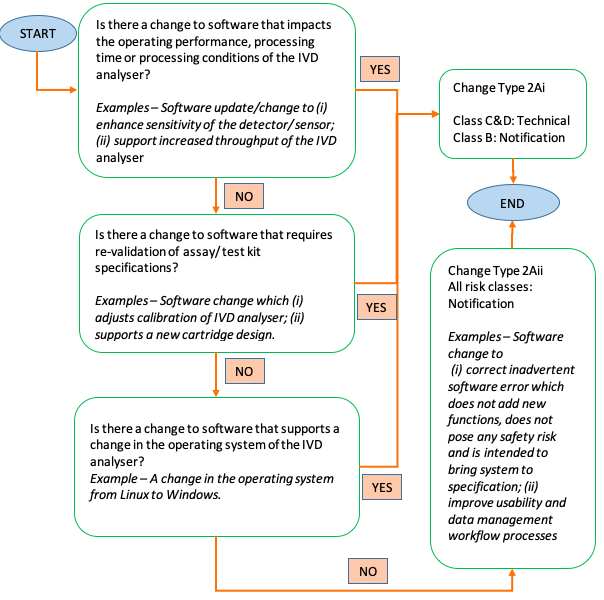
January 10, 2020 IVD Software Changes For both GN-21 and GN-34, HSA added guidance regarding notification of software changes in In Vitro Devices (IVD) illustrated in Figure 1: GN 21: Change Notification Bundling of Notification Changes Notification bundle is the major addition in the draft guidance. Under this proposal, notification changes may be bundled together to HSA in one change notification application. While bundling different notification changes, HSA reminded companies to maintain relevant inventory records to ensure traceability. In addition, the notification changes to be bundled should be submitted within 6 months after the product owner became aware of the changes. Bundling of notification changes is however not applicable to the following products: 1.) Al-based devices 2.) Changes to the drug substance/medicinal product of combination products 3.) AE/FSCA related changes Other Changes Other highlighted changes in the guidance are as follows: 1.) Finalized device label with the changes should be submitted Letter of Authorization is an added requirement for Type 6Aiv notification of Class B- D devices (Addition of New Medical Devices to a Medical Listing)
GN 34: IVD Analyzers HSA reworded a few sections of the document to clarify the guidance on IVD Analysers. Here are some of the items that have been rephrased: 1.) The Definition of Accessory and In-Vitro Diagnostic products 2.) SMDR Listing options for closed system IVD Analysers (Section 4)
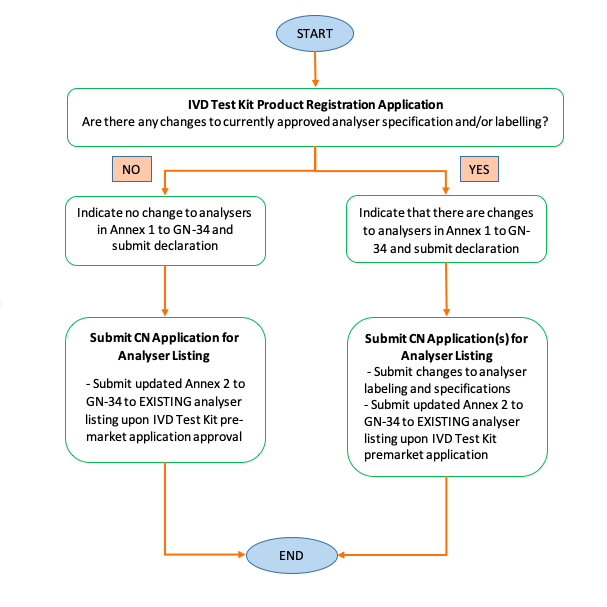
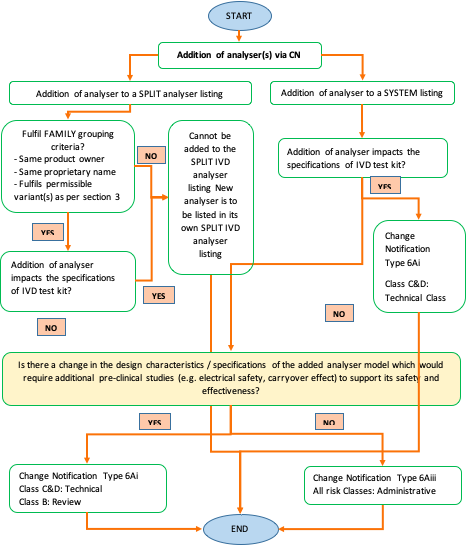
Change Notification involving IVD Analyzers In addition to GN 21, HSA reiterated some specific change notification guidance for IVD Analysers in this revised document: 1.) Guidance on adding new closed system IVD analysers to existing IVD Analyser Listings 2.) Revision of Product Registration Workflow for IVD Test Kits which have Compatible Closed-System IVD Analysers Currently Listed on SMDR in their own Analyser Device Listing(s) (Flowchart 4) (See Figure 2) Revision of Workflow for Applicable Change Notification Types for the Addition of Analyser to SPLIT IVD Analyser or IVD SYSTEM Listing (Flowchart 5) (See Figure 3)
Figure 1: Flowchart for Change to Software of In Vitro Devices (IVD) present in both revised GN 21 and GN 34
Figure 2: Revised flowchart on Product Registration Workflow for IVD Test Kits which have Compatible Closed-System IVD Analysers Currently Listed on SMDR in their own Analyser Device Listing(s) in GN 34
Figure 3: Revised flowchart on Workflow for Applicable Change Notification Types for the Addition of Analyser to SPLIT IVD Analyser or IVD SYSTEM Listing in GN 34
References:
|
- Home
- Newsletter
- SINGAPORE: HSA consults healthcare professionals and industry members on revised draft guidance documents for change notification and IVD analyzers - January, 2020