OUTLINE
- Market overview of medical devices
- Relevant Regulations for Medical devices
- Importation of Medical Devices into Malaysia
Deemed as one of the major healthcare hubs in Asia, Malaysia’ healthcare system continues to thrive tremendously with consistent support from the Malaysian government. Lately, the government has prioritized healthcare spending, injecting RM23bn (US$5.2bn) into healthcare in 2016 – approximately 10% of the annual budget. Malaysia’s healthcare system consists of two tiers: a state-owned universal healthcare system for national citizens runs alongside a private sector that serves more affluent citizens and international patients. Due to projected demographic shifts – chiefly Malaysia’s ageing population, increasing life expectancy and the growth of non-communicable diseases – demand for healthcare is expected to grow.
Market overview of medical devices
Malaysia procures most of its medical equipment from foreign firms, especially active & high risk-class MDs. But the Malaysian government is seeking to make the transition to high-value local medical device manufacturing, investing in diagnostic equipment and healthcare information technology. In 2016, the total trade for Malaysia’s medical device industry was approximately US$2 billion and it imported $585 million of medical devices. According to the Malaysian Investment Development Authority (MIDA), the local medical device manufacturing market in Malaysia had been worth US$ 400 million from January-August 2016 and it is still highly relying on imports.
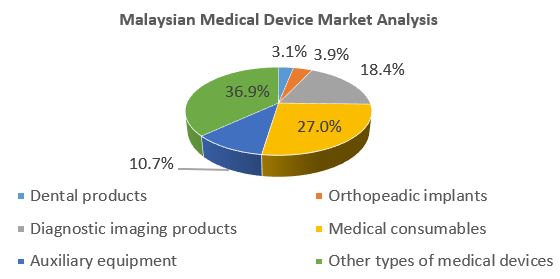
The following chart shows the major components in Malaysia’s medical device market as of 2016.
Considering Malaysia’s strong track record in electronics manufacturing, it is well-positioned to see a huge growth in its local medical device manufacturing industry. The Association of Malaysian Medical Industries (AMMI) forecasts that medical device exports will grow by 15% in 2017 (with projected revenues of RM 11 billion (US$2.5 billion)).
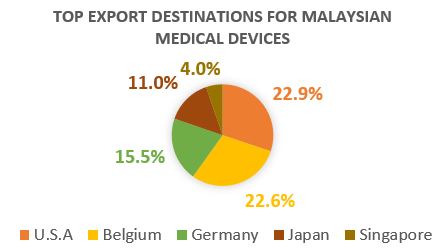
According to the statistical data published by AMMI (Association of Malaysian Medical Industry) there is a total of 159 medical equipment manufacturers in Malaysia, including 105 surgical and experimental gloves manufacturers, 10 catheter manufacturers, and 44 manufacturers that produce other medical supplies. This great growth can be clearly seen in the following chart, showing the top players in the world’s medical device industry to be key export destinations for locally manufactured medical devices in Malaysia.
Because of its long-standing rubber industry, Malaysia is the market leader in medical glove manufacturing, and is home to Hartalega Holdings and Top Glove. While the former is the largest producer of synthetic rubber gloves worldwide, the latter aims to own a 30% share in that market by 2020. The abundant resource of natural rubber is a great advantage for development of medical equipment market in Malaysia, making Malaysia an important country that exports latex products, such as surgical gloves and catheter, which account for 52% of its overall exports.
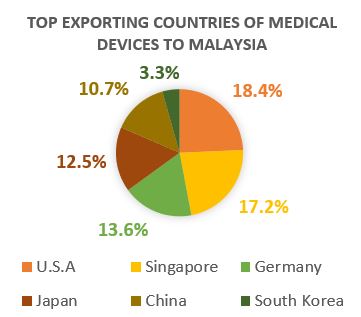
Medical device market in Malaysia is highly dependent on imports, especially in the high-end technology products. The import for medical device has increase greatly in recent years: the medical device import was approximately US$1.8 billion in 2016, which has decreased 10.7% compared to the previous year. The type of medical devices Malaysia imports and exports differ significantly. Malaysia usually imports higher classification/ category of medical devices not manufactured locally.
The major items include electronic diagnostic products and imaging equipment spare parts.
Relevant Regulations for Medical devices
The important legal rules that govern Medical Device Affairs in Malaysia are the Medical Device Act 2012 (Act 737), the Medical Device Authority Act 2012 (Act 738) and the Medical Device Regulation 2012, which were officially finalized and published in gazette, by 2014. In Malaysia, the medical device regulatory body is called the Medical Device Authority (MDA) and it is under the supervision of Ministry of Health, Malaysia. All registration of medical devices are conducted online via MDA’s online portal MEDCAST. It is important to be noted here that in Malaysia, foreign companies must appoint a Local Authorized Representative (LAR) to represent them in all official dealings with the MDA. Each medical device can only be represented by one LAR. When foreign medical device manufacturers appoint their Malaysian importers or distributors as their LARs, they have to be careful about certain possible inconveniences that might arise. If the manufacturer isn’t satisfied with the importer or distributor who’s also the device LAR, they can only proceed to transfer the responsibility if the importer or distributors cooperate willingly. Or else, they have to go through the device registration all over again, in order to change LAR. This is why, it is much easier to appoint an independent LAR for the devices, apart from having designated importers and distributors.
This is also the same case for making bids in response to Government tenders for medical device procurements in Public Healthcare Institutions. Most manufacturers tend to choose a reputable local dealer as their supply partner, in which the after-sales service and enterprise-scale are the major concerns for the bid, in addition to the product price. Furthermore, the public sector's product specifications will be handed over to the Health Technology Assessment Unit of the Ministry of Health for reasonability assessment of the bids. In Public sector procurement cases, the case of less than RM 50,000 (US$ 11, 800) is purchased through direct hospital procurement. Therefore, direct contact between local agents and hospital administrators is the most effective way to win the tendering. Larger tender with over RM 500,000(US$ 118, 807) will be performed through the Ministry of Health. For those tenders, the departments of the Ministry of Health usually publish the invitations and related information on local newspaper, in Malay dailies only if the invitees are to be local or in Malay and English dailies if they invite international biddings. The price and scope of the equipment also fluctuates every year according to annual demand. Private hospitals make purchase through their own procurement department or through external consultants. In private sectors, although some hospital chains also purchase items through centralized procurement process, but the managers of individual hospitals are usually the major decision makers of the procurement. As a result, building good relationships with them is very important.
Importation of Medical Devices into Malaysia
Malaysia applies two systems of tariff classification on the importation and exportation of goods, one for trade inside ASEAN and the other, the Harmonized System, for trade with other countries up to the six figure level. The Customs classification of goods is based on the International Nomenclature of the Harmonized System. Once obtaining the relevant Certificate of Registration from MDA, all medical devices (goods) to be imported, whether or not subjected to import duties, must be declared in writing on Customs Import Form No. 1 (Borang K1). All declarations should indicate a full and true account of the number and description of goods, packages, value, weight, measurement or quantity, and the country of origin or the final destination. Declarations must be submitted to the Customs station at the place where the goods (medical devices) are to be imported. All duties/custom taxes imposed on imported goods will need to be paid in advance by electronic fund transfer before the Customs Office authorizes the export or the import of a good.
To import the goods into Malaysia, the documents required by the Royal Malaysian Customs from the applicant include Custom Entry Form, bill of lading/airway bill, commercial invoice or Pro-forma invoice, packing list, Certificate of Registration issued by MDA, and any other relevant permits, licenses or certificates. In Malaysia, the import duties are based on the cost of the goods (on the market) and the costs of insurance, freight, commission, and other charges linked to the buying and transporting of the goods. The importer has to pay certain Customs Duties and Taxes on imports which includes 7.7% of Average Customs Duty (excluding agricultural products) and 6% of Goods and Services Tax (GST). Furthermore, qualifying goods originating from China, Japan, Korea, Pakistan, Australia, New Zealand, India, Chile and ASEAN countries imported into Malaysia may enjoy preferential rates of duty under the relevant free trade agreements.
The Ministry of International Trade and Industry (MITI) is the Ministry responsible for trade facilitation in Malaysia. For more information on the work flow of a full container load importation into Malaysia via the one of the main shipping ports, Port Klang, please visit the Ministry’s webpage.