MoH has enacted a new regulation, Permenkes No. 62 Year 2017, concerning Marketing Authorization of Medical Devices, In Vitro Diagnostic Medical Devices and Household Health Supplies on January 12, 2018. The background of the changes of Permenkes No. 1190 of 2010 concern Marketing Authorization of Medical Devices and health household supplies (PKRT) to Permenkes. 62 Year 2017 on Marketing Authorization of Medical Devices, Medical Devices DIV, and health household supplies (PKRT) are as follows:
1. PMK 1190 Th 2010> 7 years -> need to adjust to the current situation and condition
2. XV Economic Policy Package mandates simplification of Import Export Trade regulations -> acceleration service appointments
3. The development of harmonization of ASEAN-level regulation and global about Medical Devices
4. Legal Needs
The differences in Permenkes N0.62 in 2017 are as follows:
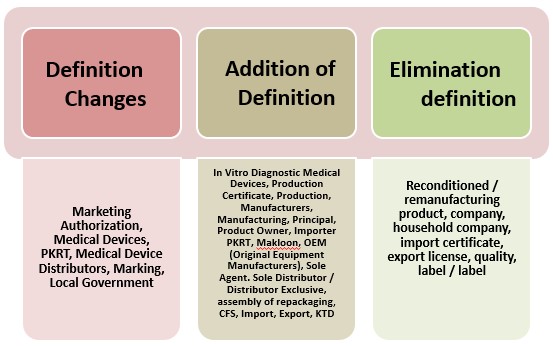
- Amendment to General Provisions on Permenkes No 62 Year 2017
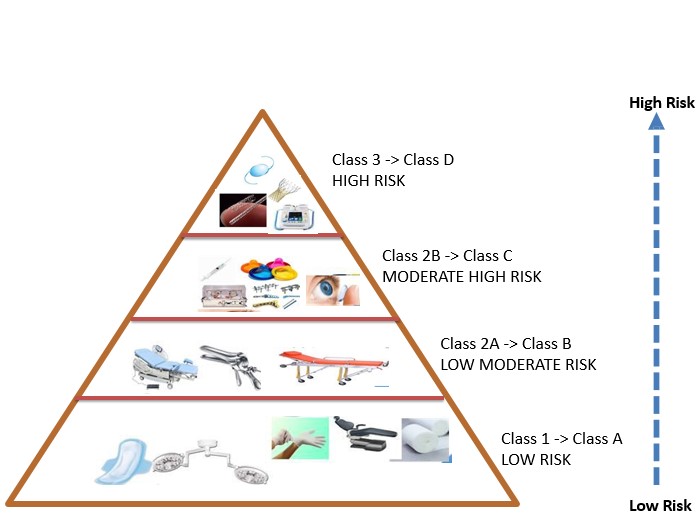
- Changes Classification of Medical Devices
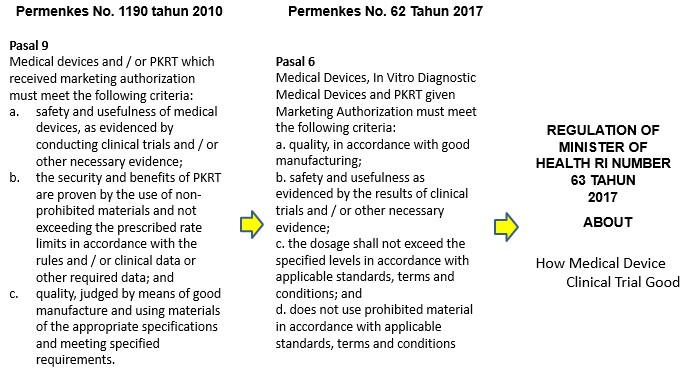
- Clinical Tests Medical Devices
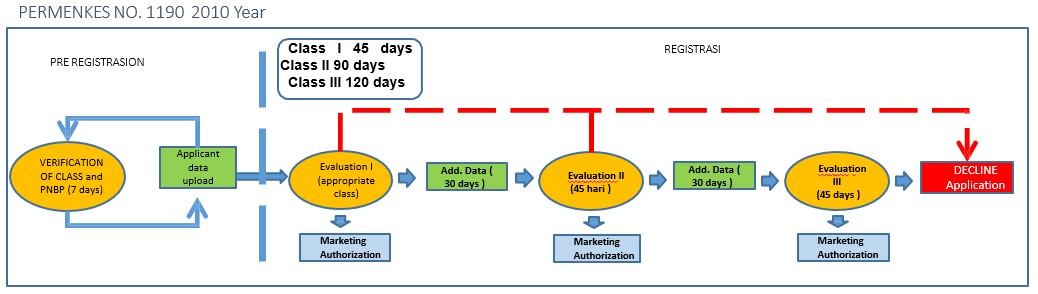
- Amendment Appointment Of The Marketing Authorization Service
The evaluation time will be shorter and so will the time required if there is additional data. There is only one chance for additional data
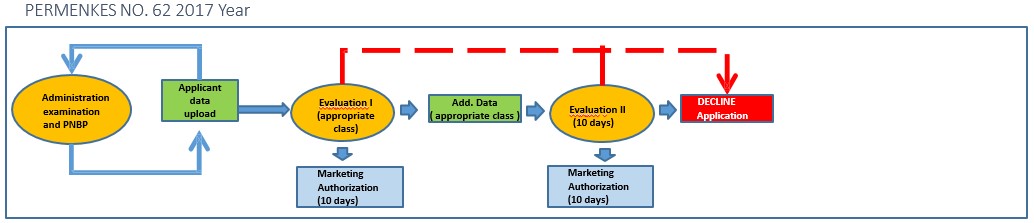
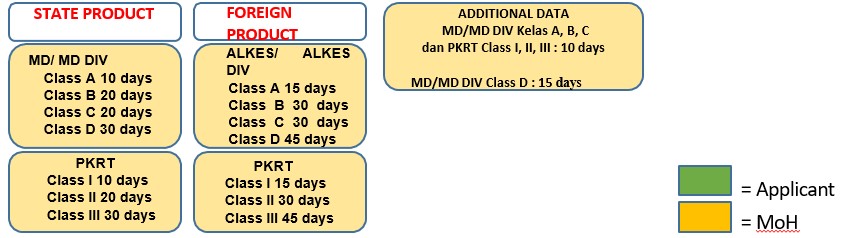
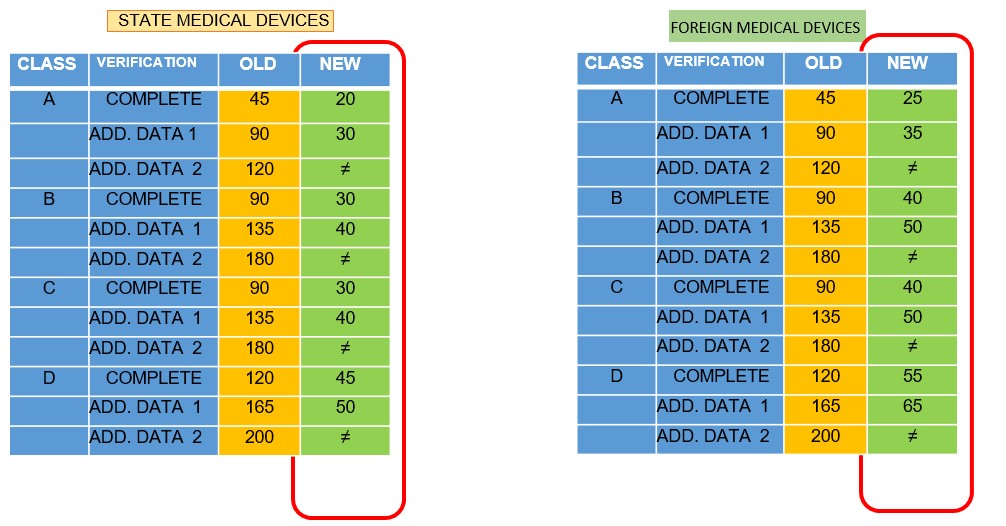
- Validity period Marketing Authorization

- Terms of extension of marketing authorization
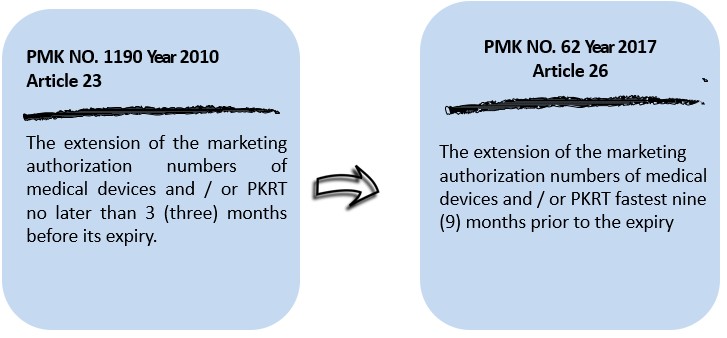
- Transition and / or termination of agency
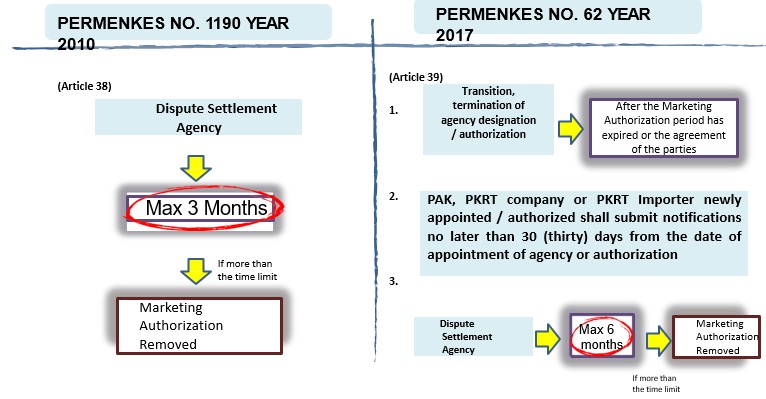
- Violation of the Regulation of MoH marketing authorization

- Arranged related types of products that can be handled
PMK No. 62 Year 2017
Article 13
Each type of Medical Device, In vitro Diagnostic Medical Device and import PKRT with 1 (one) trade name / brand originating from the Manufacturer or Principal can only be diagenkan by 1 (one) PAK or 1 (one) Importer of PKRT
- Setting up OEM Products
PMK No. 62 Year 2017
Article 26, paragraphs 3 and 4
- For Marketing Authorization of Medical Devices, In Vitro Diagnostic Medical Devices and PKRTs produced through imported OEMs may only be extended 1 (one) time.
- Import OEM circulation permit extension for Medical Devices, In Vitro Diagnostic Medical Devices and PKRT as intended in paragraph (6) shall be conducted through a review by considering the ability of domestic industry to produce similar products. - .
- Forms and other information related to the issuance and access of marketing authorization
PMK No. 62 Year 2017
Article 22 paragraphs 1 and 3
- Marketing Authorization issued in electronic form, does not require a stamp and wet signatures.
- Marketing Authorization may be printed by applicants or other interested agencies through the Indonesian National Single Window portal or regalkes.kemkes.go.id website
References:
http://regalkes.depkes.go.id/index.php/home/fileDownload/BERITA_FILE.pdf/1000287
