Post-Market Surveillance
Due to the pre-market clinical data for high-risk / innovative medical devices is relatively small, competent authorities selectively issue medical material licenses and require manufacturers to conduct clinical follow-up after listing.
Post-marketing clinical tracking is mandatory for the following types of medical devices:
• innovative medical equipment;
• High-risk medical equipment;
• medical devices affected by changes in their safety or performance;
We have extensive post-marketing clinical tracking experience, and work closely with manufacturers, agents, medical institutions and competent authorities. In recent years, our service products include hyaluronic acid subcutaneous implants, proton therapy systems, abdominal aortic vascular stents, and more High-risk medical materials in the field.
We can provide post-market clinical follow-up services, formulate post-market clinical follow-up plans based on your products, and assist you in collecting post-market clinical data.
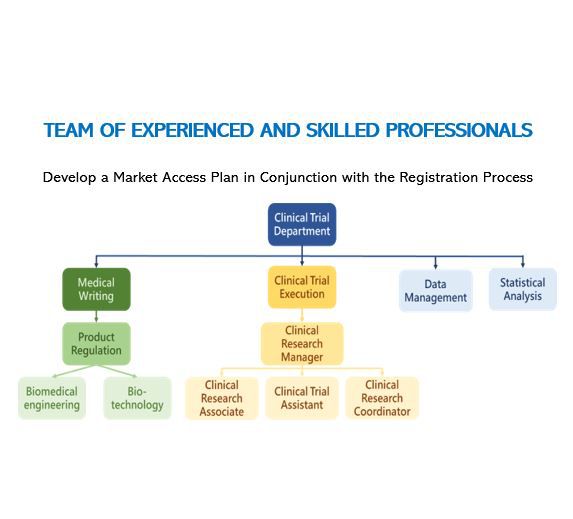
Clinical Evaluation
The clinical evaluation of medical devices is to compare the technical, biological, and clinical aspects of registered products and marketed products by registered applicants through preclinical test reports, in vitro animal experiment results, clinical literature, clinical experience, and clinical trials. The similarity between registered products and listed products in terms of use requirements, scope of application, etc., and evaluation of the effectiveness and safety of registered products.
According to your requirements, we provide the services of writing clinical assessment report in the European Union, China, Singapore and other regions.
The services are as follows:
• Similar products search
• Literature search of major databases at home and abroad
• Database search for major adverse events after listing at home and abroad
• Integration of clinical literature and non-clinical data
• Writing a clinical evaluation report

Clinical Trials
Clinical Trials is the process of confirming / verifying the safety and effectiveness of medical devices intended for market registration under normal use conditions in a medical device clinical trial institution that meets the requirements of good clinical trial specifications. Conduct clinical trials of high-risk or innovative medical devices as required by local health authorities.
We provide the following services to meet your needs:
• Clinical trial planning
• Prepare documents and send documents to the competent health authority
• Prepare and submit documents to the Human Test Committee
• Execution of Clinical Trial
• Appoint professionals to follow up clinical trials on site (CRC / CRA)
• Clinical trial data processing, Biostatistical analysis
• Write clinical trial reports, etc
We Successfully Conduct Clinical Trial for Several Medical Device
WHY CHOOSE QUALTECH?
An Excellent Management System to Ensure Time-efficient Project Handling.
Qualtech Operates Locally through our Subsidiaries in Every Serviced Country.
Expert Teams with Extensive Experience to Strongly Support Your Project.
We collect your browsing history through cookies to understand how you use our website to analyze and improve your experience. By continuing to use our website, you accept our use of cookies.