Among its ASEAN peers, Singapore spends the most annually in healthcare on a per capita basis. This is expected to rise faster, given the country's aging population and changes in demographics. Qualtech is your ideal partner to establish your company into the Singapore medical device market for an easy and smooth registration process.

Qualtech in Singapore
Experienced Team
Our team is experienced in obtaining approvals for various devices such as contact lens, facial filler, adhesive gel, software medical devices, active devices, etc. With a deep understanding of HSA reviewer’s comments.
Market Access Strategy
Qualtech Singapore team offer clients ONE-STOP market access service, covering Regulatory Research, Registration, as well as Importation, and Distributor connection.
Singapore’s HSA as Stringent Regulatory Authority
HSA is recognized as a WHO stringent regulatory authority for high risk IVD devices. Qualtech will assist you to obtain HSA approval as a springboard for faster access to various markets globally.

Medical Device Registration
Our in-house experts are able to provide you with excellent professional service in preparing ASEAN Common Submission Dossier Template (CSDT) and liaise with HSA to get your devices registered in the Singapore.

Authorized Representation
As an in-country authorized representative (AR), Qualtech can hold a medical device registration license on behalf of foreign manufacturers looking to market medical devices in the Singapore. This is in compliance with the law for a local establishment to be a license holder.
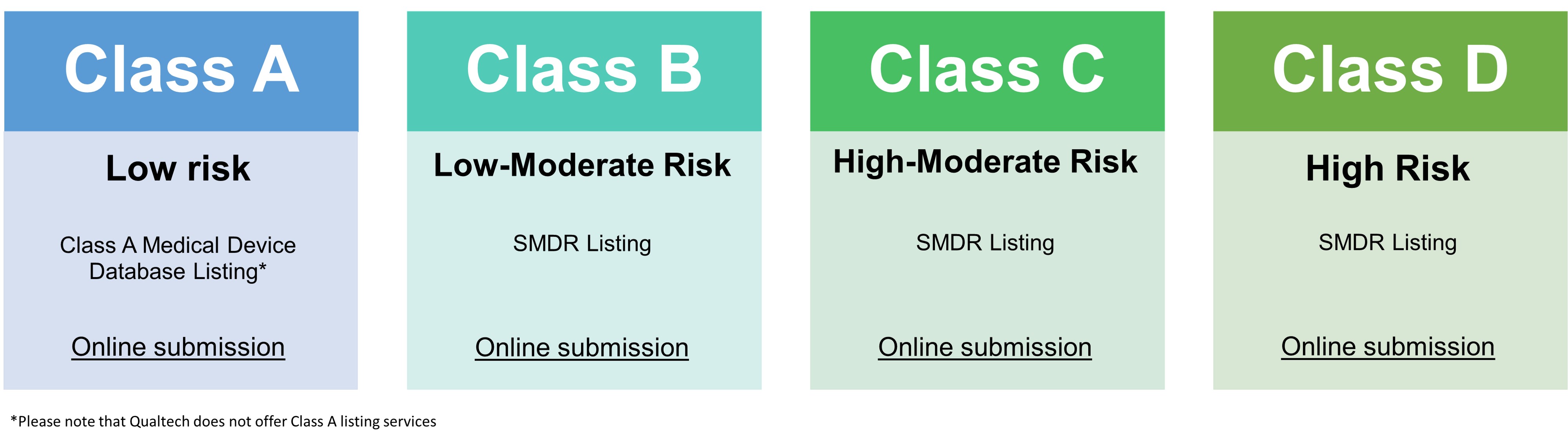
| Registration with HSA | |
|
 |
CLASS B | CLASS C | CLASS D |
|---|---|---|---|
| Executive Summary | Yes | Yes | Yes |
| Essential Principles and Methods | Yes | Yes | Yes |
| Device Description | Yes | Yes | Yes |
| Design Verification & Validation | Yes | Yes | Yes |
| Labeling and IFU | Yes | Yes | Yes |
| Risk Analysis | Yes | Yes | Yes |
| Manufacturer Information | Yes | Yes | Yes |
| Clinical Evidence Report | Yes | Yes | Yes |
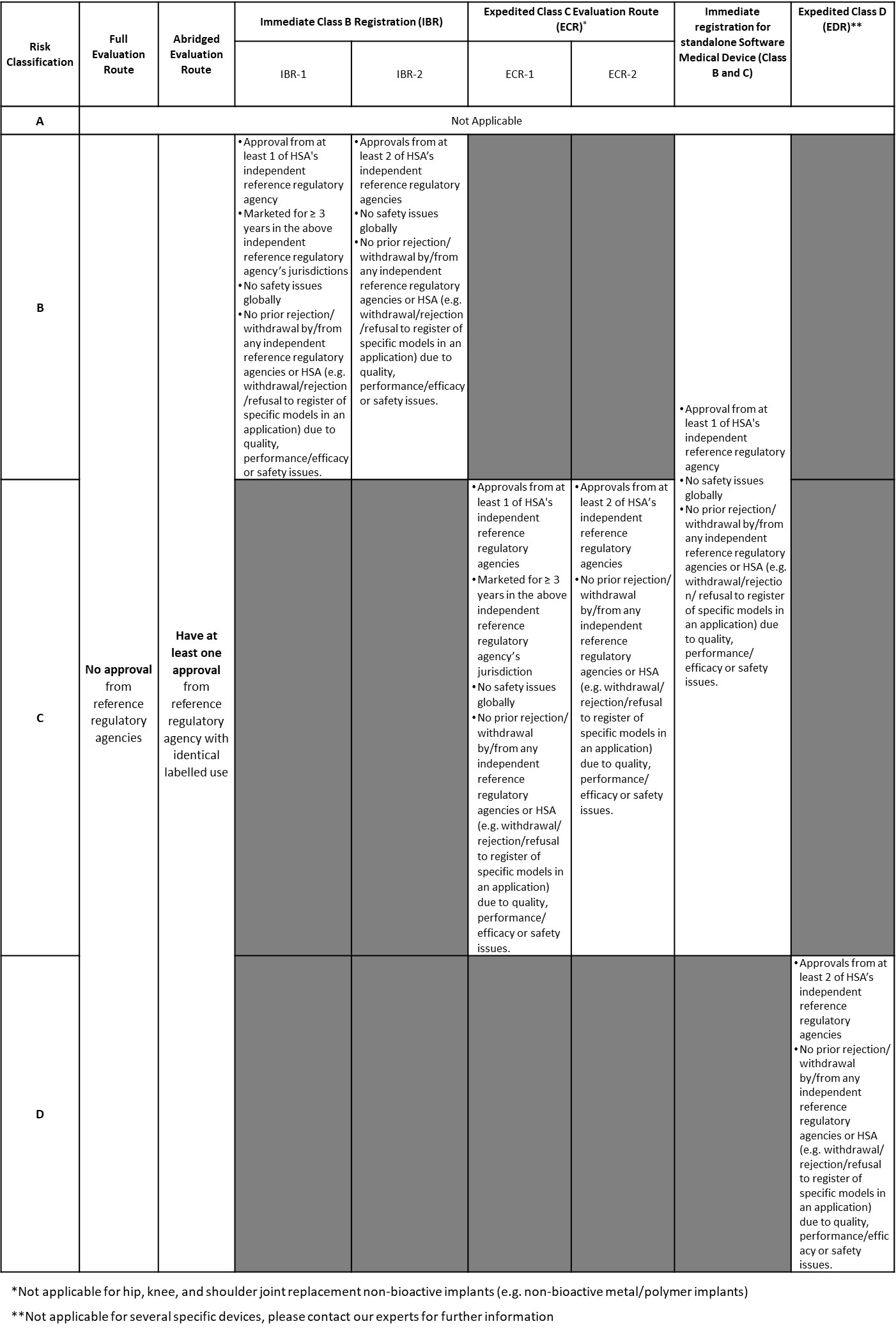
HSA Target Reviewing Turnaround Time
After successful internal evaluation of the submission dossier, our in – house experts will then submit it to the HSA and will be subjected to thorough evaluation. HSA will review within the promised turnaround times based on the class and type of registration route.
|
Registration Route |
Class B |
Class C |
Class D |
Class D with a registrable drug |
|
Immediate route |
Immediate upon submission |
-
|
-
|
|
|
Expedited route |
-
|
120 |
180 |
-
|
|
Abridged route |
100 |
160 |
220 |
220 |
|
Full route |
160 |
220 |
310 |
310 |
|
Full route (Priority Review Scheme Route 1) |
120 |
165 |
235 |
-
|
|
Full route (Priority Review Scheme Route 2) |
120 |
165 |
235 |
-
|
- Note:
-
*This data is accessed on 6 July 2023 from HSA’s official website
**Kindly refer to the official website for updated info
-
-
An annual retention fee is payable in order to retain the registration of the medical device on the SMDR.
Qt has established a stable status in Singapore and will ensure hassle-free annual renewal process of your product’s registration.
|
Singapore is known as medical and healthcare-hub in ASEAN as the country draws approximately 500,000 patients each year. Offering Asia's best healthcare system, Singapore is a promising and well-known market for medical devices. It's good news that HSA offers several faster access routes with shortened timelines, enabling foreign manufacturers to have immediate and faster market access. HSA allows several evaluation routes with shortened timelines enabling faster market access, such as immediate, expedited, and abridged route. These routes apply to medical devices that have been evaluated and have obtained marketing clearances or approvals in at least one of the Global Harmonization Task Force (GHTF) founding members (Australia, Canada, European Union, Japan and United States of America). Consult with our regulatory expert to know if your device is eligible for any faster route. In June 2023, HSA proudly announce that they are acknowledged by WHO as a Stringent Regulatory Authority for high-risk IVD devices. This is good news for global manufacturers, as this status means obtaining approval from HSA will result the product to be applicable to gain expedited listing under the WHO prequalification programme. With note that major international purchasers, e.g., the United Nations agencies rely on this listing. For more information, please refer to the Singapore HSA's official website or you may contact us for a free consultation |
|
|
Official Website
|
|
★ HSA Website Homepage |
We collect your browsing history through cookies to understand how you use our website to analyze and improve your experience. By continuing to use our website, you accept our use of cookies.
