Singapore has long been dubbed as the heart of MedTech in Asia. Being a small island country, it is a competitive economic hub for the region in terms of ease of doing business, best labour force, strong IP protection and being Asia’s leading logistics hub.
Although Singapore is the smallest country in ASEAN, it is also having the most advanced healthcare service industry. The public healthcare system is very developed and the public healthcare institutions are well-managed and well-funded. It strives to use the latest medical healthcare technologies. These institutions and their private sector peers are therefore influential customers whose purchasing-power and decisions are often seen as a meaningful endorsement in Asia and beyond. This is further endorsed by a report recently published by market insights firm Fitch Solutions, stating that Singapore’s healthcare market is expected to grow to S$29.8 billion in 2020. Hence, 9% more than last year’s S$27.3 billion, and well over double to S$68.7 billion by 2029.[1]
Risk Classification and Submission Procedure
Medical devices in Singapore are regulated under the Health Products Act (HPA) and subsequently the Health Products (Medical Devices) Regulations 2010. HPA requires that the product owner and/or its local registrant must register any medical device with the local governing body named, Health Science Authority (HSA), in order to be listed in the Singapore Medical Device Register (SDMR). There are four risk classifications followed in Singapore for all products falling under the definition of a medical device, namely Class A, B, C and D.
Table 1: Overview of HSA risk classifications and Evaluation Routes
|
Class |
Risk Level |
Device Examples |
Evaluation Routes Available |
Basic prior approval requirements |
|
A |
Low Risk |
Surgical retractors / tongue depressors |
Directly list the device in Class A Medical Device Registry |
|
|
B |
Low-moderate Risk |
Hypodermic Needles / suction equipment |
Full Evaluation Route |
No prior approval in any of HSA’s independent reference regulatory agencies |
|
Abridged Evaluation Route |
At least ONE prior approval in any of HSA’s independent reference regulatory agencies |
|||
|
Immediate Class B Registration (IBR) Evaluation Route |
~At least ONE prior approval in any of HSA’s independent reference regulatory agencies ~Marketed for 3 years in the said agency’s jurisdiction |
|||
|
C |
Moderate-high Risk |
Lung ventilator / bone fixation plate |
Full Evaluation Route |
No prior approval in any of HSA’s independent reference regulatory agencies |
|
Abridged Evaluation Route |
At least ONE prior approval in any of HSA’s independent reference regulatory agencies |
|||
|
Expedited Class C Registration (ECR) Evaluation Route |
~At least ONE prior approval in any of HSA’s independent reference regulatory agencies ~Marketed for 3 years in the said agency’s jurisdiction |
|||
|
Immediate Class C Registration (ICR) Evaluation Route |
For standalone medical mobile application only, with same requirements as ECR route |
|||
|
D |
High Risk |
Heart valves / implantable defibrillator |
Expedited Class D Registration (EDR) Evaluation Route |
At least TWO prior approval in any of HSA’s independent reference regulatory agencies |
Generally, a product registration application must be prepared in accordance with the ASEAN Common Submission Dossier Template (CSDT) in English and must be accompanied by all relevant certificates, reports, and copies of labelling etc., as annexure. Depending on the evaluation route chosen by the owner/registrant, the extent of details required will vary. However, this is not applicable for Class A sterile and non-sterile medical devices, which can be directly listed in the Class A Medical Device Registry without undergoing any evaluation.
Submission of application is done with HSA by a company that is registered with Accounting and Corporate Regulatory Authority (ACRA). It may be the Singapore subsidiary of the product owner (principal manufacturer) or a local company that is authorized by the product owner to submit the product registration application.
Singapore’s Soaring Medical Device Landscape
Singapore has a rapidly aging society, caused by increased life expectancy coupled with decreasing birth rates. By 2030, the nation will join 33 others as a “super-aged” country.[3] Owing to this, the landscape of Singapore’s medical device market caters to these targeted societal ailments. Fitch Solutions predicts Singapore's medical device market to grow by 8.4 per cent in 2020, 2% more than the previous annum.[2]
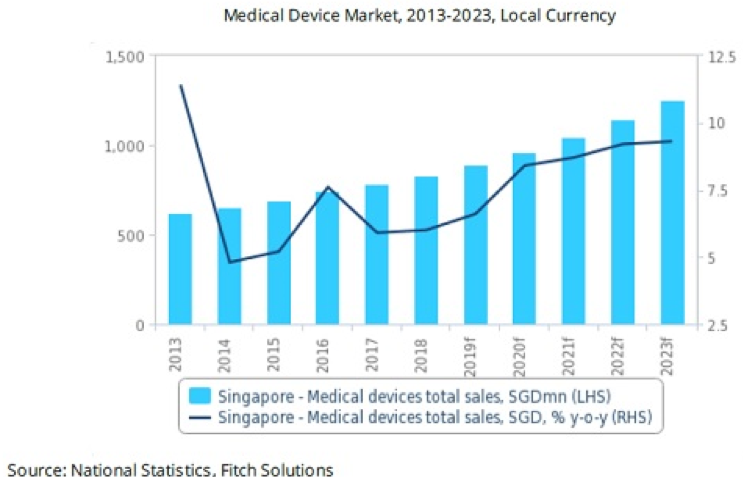
Figure 1: Singapore's medical device market value to hit $1.3b by 2023
According to the research house, market drivers include a rapidly ageing population with a growing disease burden, high quality healthcare provision financed by a combination of private saving schemes and government subsidies, as well as a well-developed medical tourism industry. Furthermore, strong government financial backing for the healthcare sector, an expanding medical device industry attracting multi-national investment, ongoing regulatory improvements, and new free trade agreements should also drive sector growth, the report stated. Separately, it added that medical device imports will benefit from increased government spending on healthcare capacity and new medical technologies.
With a rapidly ageing population, Singapore will need more assistive medical technologies and therapeutics. There is an immediate market for products in population health management, which includes tele-health, tele-monitoring and other healthcare informatics solutions which aim to alleviate the pressures of Singapore’s ageing population.
There is also a huge demand for medical device solutions that seek to manage obesity-related and chronic diseases, tapping into demographic and lifestyle trends of increasing incidences of hypertension and diabetes. Furthermore, manufacturers offering solutions in digital dentistry will be able to find opportunities amidst rising interest in this field.
Table 2: In demand product sectors in Singapore from 2015 to 2020
|
Product Type |
CAGR (2015-2020) |
|
Orthopaedics & Prosthetics |
11.9% |
|
Other Medical Devices |
11.7% |
|
Patient Aids |
11.7% |
|
Consumables |
10.8% |
|
Diagnostic Imaging |
9.1% |
|
Dental Products |
8.8% |
Talking of another market driver, the government’s strategies to transform the healthcare sector have yielded results, as Singapore is being hailed as one of the best medical tourism hubs in the ASEAN region. Singapore serves as a showcase for new medical technology and healthcare delivery, attracting more than 500,000 medical tourists annually who account for just under 4% of overall tourism receipts (US$1 billion – Interestingly, 60% of these were Indonesian patients).[3] Despite facing increasing competition, Singapore will continue to be the preferred destination in specialized areas of medicine such as oncology, organ transplants, orthopaedics, cardiology, and neurology, among others.[4]
Importation of Medical Devices into Singapore
A company importing medical devices into Singapore is required to hold an importer’s license. Application must be accompanied by certificate of Good Distribution Practice for Medical Devices in Singapore (GDPMDS) or ISO 13485 certificate, with scope of storage and distribution included and a list of exempted Class A medical devices imported. A License is valid for 12 calendar months, and an email notification reminder for license renewal will be sent from the Authority to the licensee 60 calendar days before the expiry of license. Application for renewal must be submitted 40 calendar days prior to the expiry date of license. The importers have the following mandatory obligations to fulfill:
- Maintain records of import and supply
- Maintain records of complaints
- Report defects and adverse effects to HSA
- Notify to HSA concerning field safety correction actions
- Follow prohibition against false or misleading advertisement
Conclusion
As Singapore continues to dazzle as a major Asian medical hub, we may help you seize the opportunity to enter Singapore’s market by registering your medical devices, as well as assisting you to connect with local distributors – ultimately, leading you to a smooth journey towards the expansion of your products into the Singaporean market.
References:
- Singapore healthcare market set to grow to S$29.8b this year: Fitch Solutions
- Growth in Singapore medical device market cut to 8.4% for 2018-2023: Report
- Singapore’s Healthcare Industry: Gateway to ASEAN’s Healthcare Market
- A MEDTECH BOOM IN SOUTHEAST ASIA
- TAPPING ON ASEAN'S HEALTHCARE OPPORTUNITIES THROUGH SINGAPORE
- Chart of the Day: Singapore's medical device market value to hit $1.3b by 2023
- Market Opportunity: Singapore
