Fueled by increasing demand for healthcare services, and emergence of new care models beyond traditional hospital settings, Malaysia is fast growing to be ASEAN's largest medical device hub. Qualtech is your ideal partner to for your foray into the Malaysia medical device market for an easy and smooth registration process.

Qualtech in Malaysia
GDPMD Certificate
We are a Malaysia Government approved entity to process regulatory and distribution work of medical device. We have successfully assisted foreign manufacturers to access local market.
200 Cases Approval
There are over 50 manufacturers entering Malaysia market with Qualtech local team support. We especially offer an efficient registration way for high risk products to open local market in a short time.

Medical Device Registration
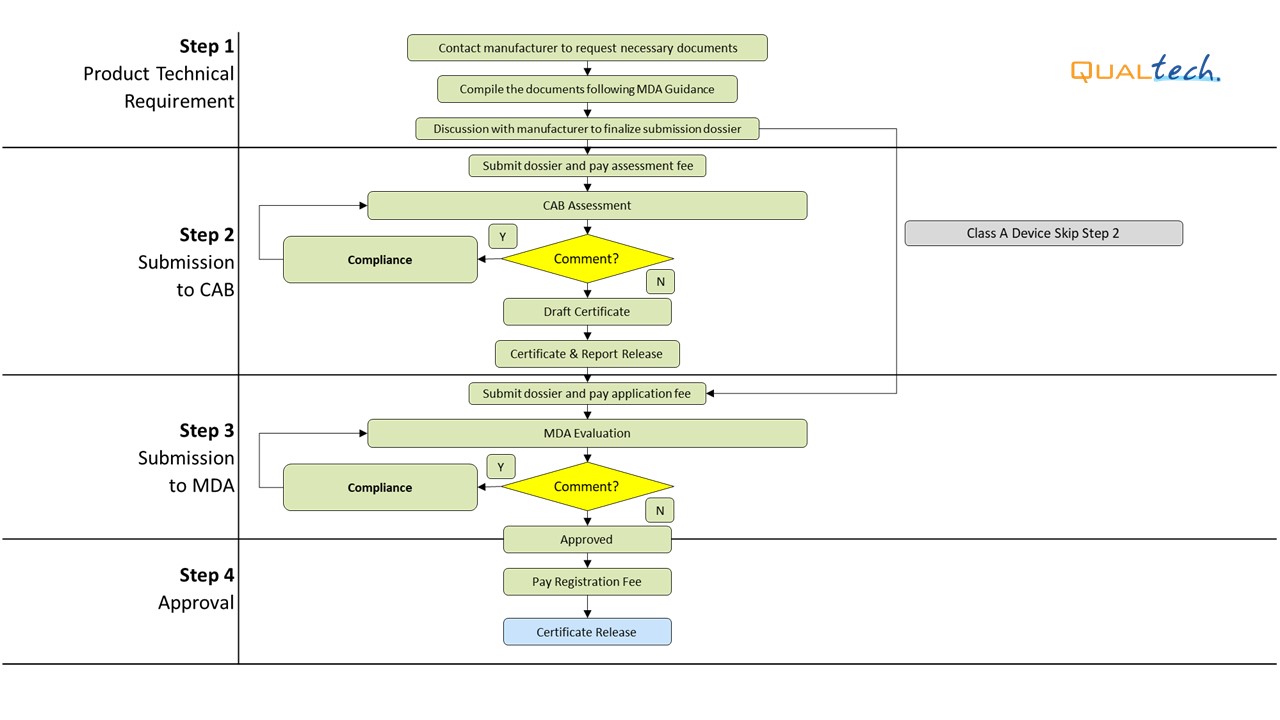
Our in-house experts are able to provide you with excellent professional service in preparing customized ASEAN Common Submission Dossier Template (CSDT) and liaise with MDA officers to get your devices registered in the Malaysia.

Authorized Representation
As an in-country authorized representative (AR), Qualtech can hold a medical device registration license on behalf of foreign manufacturers looking to market medical devices in the Malaysia. This is in compliance with the law for a local establishment to be a license holder.
Importation
The local distributor you are looking is sometimes not a qualified importer, or is not familiar with the importation process of medical device. Qualtech has extensive experience in importation in many regions of Asia, assisting several customers to handle customs clearance of various medical equipment imports. We can assist your products to be delivered to customers smoothly!

Establishment License Application
We also provide professional guidance and service for local manufacturers, importers, distributors and authorised representatives to apply for an Establishment License with MDA. Having a team of local consultants who are well-versed in the requirements of local regulations and MEDCAST system, your journey will be smooth with us.
|
Registration with MDA |
|
|
 |
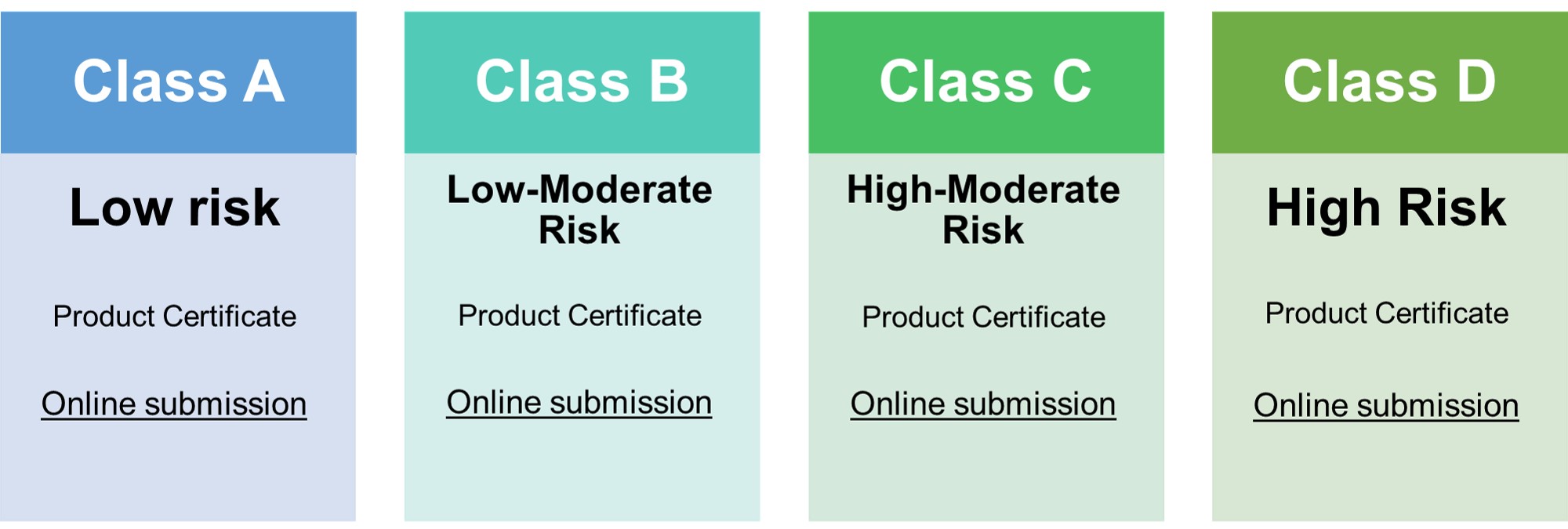
CLASS A | CLASS B | CLASS C | CLASS D |
|---|---|---|---|---|
| Executive Summary | Yes | Yes | Yes | |
| Essential Principles and Methods | Yes | Yes | Yes | |
| Device Description | Yes | Yes | Yes | Yes |
| Summary of Design V&V | Yes | Yes | Yes | Yes |
| Labeling and IFU | Yes | Yes | Yes | Yes |
| Risk Assessment | Yes | Yes | Yes | |
| Physical Manufacturer Information | Yes | Yes | Yes | Yes |
| Clinical Evidence | Yes | Yes | Yes | |
| PMS Plan and Report | Yes | Yes | Yes | |
| QMS Audit Report | Yes | Yes | Yes | Yes |
Note: Kindly see the MDA/GD/0008 COMMON SUBMISSION DOSSIER for more details.
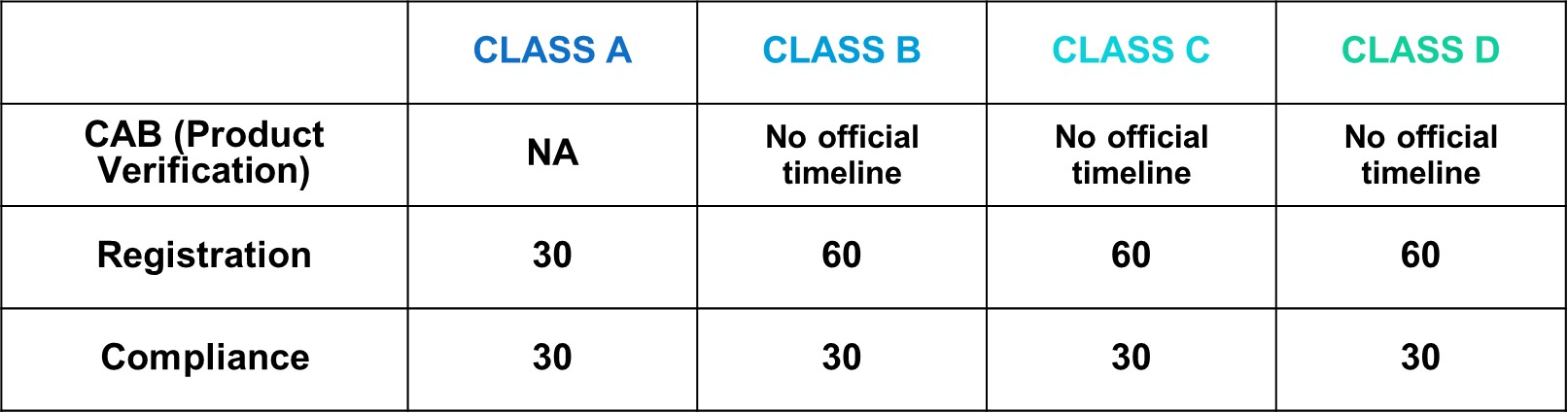
MDA Target Reviewing Evaluation Time
After successful internal evaluation of the submission dossier, our in-house experts will then submit it to the MDA and will be subjected to thorough evaluation. Below is a list of the evaluation times each round for different types of transactions involving product registration/notification.
- Note:
- 1. It's accounted as Working days.
- 2. CAB in Full Assessment would take around 8 months.
-
Types of Certificates: Medical Device Registration Certificate, CAB Certificate
-
Medical Device Registration Certificate: 5 years
-
CAB Certificate(product verification certificate & full assessment certificate): 5 years
All foreign manufacturer would like to have their product access Malaysia market immediately. It's a good news that MDA offers a route of faster market access to shorten the registration timeline.
For the Class B, C and D product:
If the manufacturer holds a product registration certificate from any of below reference countries, MDA-recognized CABs allow product conformity assessment via verification routes with shortened timelines, enabling faster market access.
MDA-recognized reference markets, including Australia, Canada, European Union countries with CE mark, Japan, United Kingdom (MHRA), and the United States. The manufacturer shall provide the local product certificate to verify the product is allowed on market.
For more information, please refer to the MDA’s official website or you may contact us for a free consultation.
We collect your browsing history through cookies to understand how you use our website to analyze and improve your experience. By continuing to use our website, you accept our use of cookies.
