Strengthening International Regulatory Collaboration
The Ministry of Health (MOH) Malaysia has launched the Medical Device Regulatory Reliance Programme with China, marking a historic advancement in international regulatory collaboration. The collaboration allows Medical Device Authority (MDA) and China's National Medical Products Administration (NMPA) to recognize each other's regulatory decisions for pre-market approvals. Malaysian IVD will gain access to China's Green Channel, and Chinese IVD devices will have a benefit to use Malaysia's Verification Pathway, as a result, the approval timelines can be reduced to 60 and 30 working days respectively. This reliance programme will officially be implemented from 30th July 2025 to 30th September 2025.
Eligibility Criteria for Chinese-Made IVD Devices (Malaysia's Verification Pathway)
As shown in Figure 1, there are several eligibility criteria for IVD devices to qualify for Malaysia's Verification Pathway and China's Green Channel. For Chinese-Made IVD devices seeking registration under Malaysia's Verification Pathway, the eligibility criteria are:
- The manufacturer must be located in China.
- The manufacturer must own and operate its facility in China
- Third-party brand owners (e.g., rebranders, relabelers, assemblers) are excluded from participating in this programme.
- The IVD medical device must be classified as Class II (approved by the provincial MPA) or Class III (approved by the NMPA).
- Priority will be given to applications involving rare diseases and innovative medical devices.
- A maximum of 6 applications.
- Contact via cab.registration@mda.gov.my
Eligibility Criteria for Malaysian-Made IVD Devices (China's Green Channel)
For Malaysian-Made IVD devices seeking registration under China's Green Channel, the eligibility criteria are:
- The manufacturer must be located in Malaysia.
- The manufacturer must own and operate its facility in Malaysia.
- Third-party brand owners (e.g., rebranders, relabelers, assemblers) are excluded from participating in this programme
- The IVD medical device must be classified as Class B, C, or D (approved by the MDA)
- A maximum of 6 applications
- Contact via info@ChinaMedDevice.com
Application Process for Chinese-Made IVD Devices in Malaysia (Pilot Phase 1)
For Chinese-Made IVD devices that are eligible for Malaysia's Verification Pathway, must follow a specific guidance under the Malaysia-China Medical Device Regulatory Reliance Programme (Pilot Phase 1). As shown in Figure 2, the manufacturer must appoint a licensed local Authorized Representative (AR) that hold a valid Establishment License issued by the MDA in accordance with the Act 737. The appointed AR shall submit the premarket documentation to MDA for screening via cab.registration@mda.gov.my. These premarket documentations include:
- Quality Management System (QMS): ISO 13485, MDSAP, QSR (FDA 31 CFR Part 820), or Japan MHLW Ordinance 169
- Medical device information: Device name, intended use, classification, rule, and grouping
- CSDT documentation
- Post-Market Surveillance (PMS)
- Declaration of Conformity (DoC)
- Class II registration certificate by provincial MPA or Class III registration certificate by NMPA
The MDA will screen the complete submission application to determine its eligibility under Pilot Phase I and appoints CAB with relevant technical code to conduct verification assessment if the application fulfills the requirements. Upon completion of the verification assessment and satisfactory review, the CAB will issue a certificate. The Authorized Representative (AR) shall proceed to make a submission of the medical device registration to MDA via MeDC@St System. Following a complete evaluation and satisfactory review, MDA will issue a registration certificate valid for 5 years and device will be listed in the Medical Device Register (MDAR) for the same duration of registration certificate validity. Figure 2 is show the explanatory notes regarding the submission of applications to MDA.
Benefits of the Reliance Programme
This collaboration able to reduce regulatory duplication, speeds up market access for eligible IVD devices in both countries, and improves patient access to quality healthcare technologies. Indirectly, it helps to strengthen Malaysia's position as a regional regulatory hub and helps to enhance global investor confidence in the country's regulatory ecosystem.
Clarification through Visuals
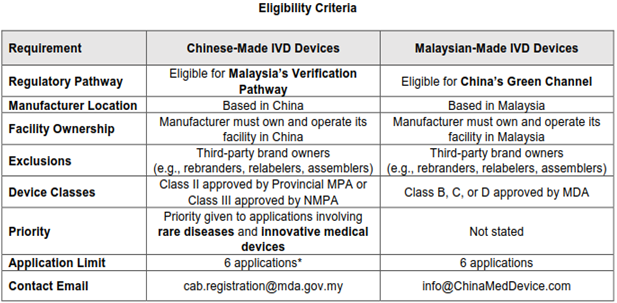
Figure 1: Eligibility criteria for IVD Medical Device in Malaysia and China
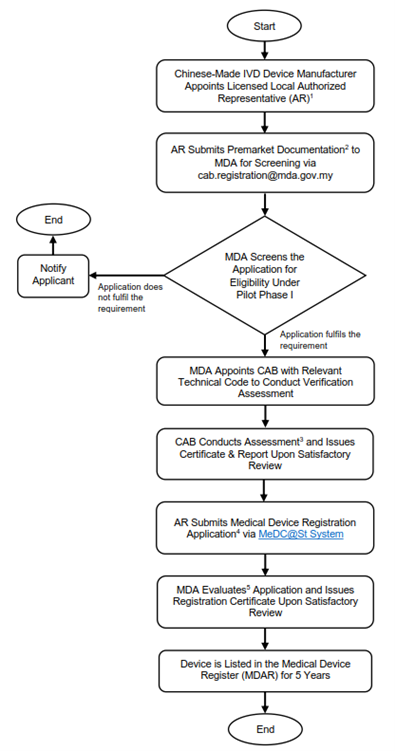
Figure 2: Submission of Class B, C and D Chinese-Made IVD Devices to MDA for Malaysian's Verification Pathway
How Qualtech Can Help
With the Malaysia–China Medical Device Regulatory Reliance Programme opening a new fast-track pathway for IVD devices, navigating the eligibility and compliance process correctly is essential.
Qualtech's regulatory experts can assist you in:
- Assessing eligibility under the Reliance Programme
- Preparing and submitting regulatory documentation to MDA or NMPA
- Acting as your local Authorized Representative (AR) in Malaysia
- Guiding you through verification and registration to secure market entry efficiently
References
- Press Release: Malaysia Leads the World in Medical Device Regulatory Reliance
- Implementation of Malaysia-China Medica Device Regulatory reliance Programme (Pilot Phase I)
