Introduction
Australia is entering a new era of medical device regulation with the rollout of the Unique Device Identification (UDI) system. Designed to enhance patient safety, traceability, and post-market surveillance, the UDI framework aligns Australia with global best practices, including the EU MDR and US FDA systems. The Therapeutic Goods Administration (TGA) introduced the UDI under the Therapeutic Goods Legislation Amendment (Australian Unique Device Identification Database and Other Measures) Regulations 2025. This article provides medical device manufacturers with a clear guide to understanding and complying with the Australian UDI requirements.
Understanding the Australian UDI Framework
What is a Unique Device Identifier (UDI)?
A UDI is a globally recognized code comprising:
- UDI-DI (Device Identifier): A fixed identifier unique to a device model.
- UDI-PI (Production Identifier): Variable data such as lot number, expiry date, and serial number. These must be issued by a TGA-recognized agency: GS1, HIBCC, or ICCBBA.
UDI Carrier Requirements
- Must appear in Human Readable Interpretation (HRI) and Automatic Identification Data Capture (AIDC) formats.
- Applied to the label, the device itself (for reusable devices), and all applicable packaging levels.
Database Obligations – The AusUDID
- Sponsors must submit and maintain UDI-DI data in the Australian Unique Device Identification Database (AusUDID).
- Each UDI-DI must be linked to an ARTG entry.
Compliance Roles and Responsibilities
Manufacturer Responsibilities
- Select an approved UDI Issuing Agency.
- Apply and label devices with UDI-DI and UDI-PI.
- Ensure proper placement and format of UDI Carrier.
- Directly mark reusable devices.
Sponsor Responsibilities
- Submit UDI records to AusUDID.
- Ensure manufacturer compliance.
- Include UDI in adverse event reports, recalls, and implant cards.
Third-Party Roles
- May apply labels or submit data but remain under the accountability of the manufacturer or sponsor.
Devices in Scope and Exemptions
Devices Requiring UDI
- Medical Devices: Class Is, IIa, IIb, III
- IVDs: Class 2, 3, 4 and certain Class 1 (software or instruments)
Exemptions
- Class I non-sterile/non-measuring
- Custom-made devices, in-house IVDs
- SAS/AP-supplied products
- Export-only and Surgical Loan Kits at kit level
UDI Compliance Timeline (2025–2030)
Key Compliance Milestones
- Milestone 1: UDI on labels and data submission to AusUDID
- Milestone 2: Direct marking for reusable devices
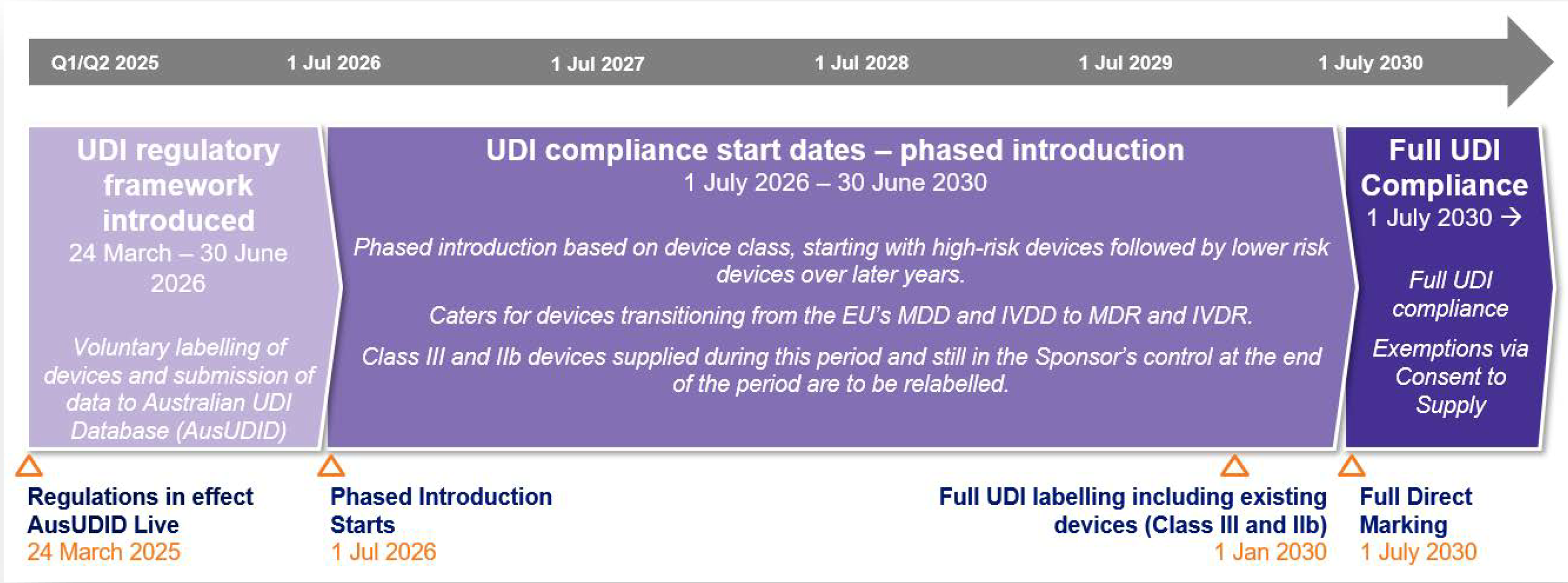
Medical Device Timeline
| Device Class | UDI Carrier on Label & Submit to database | Direct Marking & Submit to database |
|---|---|---|
| Class III, IIb | 1 July 2026 | 1 January 2028 (III), 1 January 2029 (IIb except for implantable MDs) |
| Class Iia | 1 July 2027 | 1 Jan 2029 |
| Class Is | 1 July 2028 | 1 Jan 2029 |
IVD Timeline
| Device Class | UDI Carrier on Label & Submit to database | Direct Marking & Submit to database |
|---|---|---|
| Class 3 and 4 | 1 July 2028 | 1 July 2029 |
| Class 1 and 2 | 1 July 2029 | 1 July 2030 |
EU Certificate Transition
- Devices under MDD/IVDD have extended timelines
- MDR/IVDR-certified devices must follow Australian UDI dates
Special Device Cases and Labelling Considerations
- Direct Marking
- Required for reusable devices unless exempted
- Not applicable to capital equipment
- Unit of Use (UoU) DI
- Needed when multiple unpackaged items are supplied in one base unit
- Software as a Medical Device (SaMD)
- Major updates = new UDI-DI
- Minor revisions = new UDI-PI
- Procedure Packs, Kits, Systems
- System/Procedure Pack requires UDI if at least one high-risk device is included
- Each component needs a UDI if sold separately
Recordkeeping and Reporting Obligations
- Retention periods:
- 10 years: Class III & IIb implantable MDs, and Class 4 IVDs
- 5 years: Other classes
- UDI must be included in:
- Market action reports
- Implant Cards
- Adverse event reporting
Compliance Pitfalls to Avoid
- Using an unapproved Issuing Agency
- Failing to link UDI to ARTG ID
- Not updating AusUDID within 30 days of first supply
- Missing direct marking requirements for reusable devices
How Qualtech Can Help
- UDI compliance strategy and regulatory assessment
- Data entry and AusUDID submission (Excel, HL7 SPL, or NPC methods)
- UDI labelling consultation
Australia's UDI implementation is a significant regulatory shift that will transform how medical devices are identified, traced, and monitored. Compliance is not just a box to check, it is a commitment to patient safety and market sustainability. Early action ensures smoother transition, fewer disruptions, and a stronger reputation.
Qualtech Consulting Corporation has been a trusted partner for medical device manufacturers for 25 years. Whether you're a local startup or an international player, we empower your devices to enhance lives.
Connect with us today to unlock your medical device potential.
References
- 1. Complying with the Unique Device Identification requirements for medical devices
- 2. Complying with the Unique Device Identification timeframes for medical devices
