PHILIPPINES: PFDA UPDATES DRAFT ON IVD REGULATION – September/October 2022
2022-09-27
FDA Circular No.2014-005 has been the guidance for registration of selected IVD devices from 2014 up to present. All non-listed IVD devices on the said circular have been exempted from the registration process and considered as non-registrable IVDs. To harmonize with the ASEAN requirements on IVD regulation, PFDA presented a regulation draft that aims to update the rules, guidelines, procedures, and requirements for IVD registrations during the first quarter of 2022. As an update to this document, PFDA released an updated document last September 2022 and seeks comments of the stakeholders on the proposed draft of AO, via virtual public hearing.
The following are the notable topics and changes presented at the said hearing.
|
Current Draft Provision
|
Previous Draft (1st QTR 2022)
|
|
IVD products are classified into various categories:
- IVD for Commercial Distribution
- Government Internationally Procured IVDs
- Donated Brand New IVD
- Research Use Only (RUO) IVD
- IVDs that are Not Intended for Commercial Distribution
- Imported IVD for Personal Use
Each category has different authorization requirements.
|
|
|
IVD for Commercial Distribution:
- - Authorization: All IVD classifications (A, B, C, D) will require a CIVDR.
- - Risk Classification: Based on Annex 3 of the AMDD
- - Certificate Validity: 5 years
- - Performance Validation: required for selected Class B, C and D IVDs. PFDA to publish updated list
|
Authorization: Class A IVD requires a Certificate of IVD Notification (CIVDN).
Class B, C and D IVD require a Certificate of IVD Registration (CIVDR).
Risk Classification: Based on Annex 3 of the AMDD
Certificate Validity: 5 years
Performance Validation: Required for Selected IVDs
|
|
Current Draft Provision
|
Previous Draft (1st QTR 2022)
|
|
CIVDR Automatic Renewal: Applicable if the following conditions are met:
- - Application filed before expiry date
- - Renewal Fee is paid
- - Submitted statement indicating no change in the product
- - Submitted Letter of Authorization, QMS Certificate (e.g., ISO13485) and Colored Photos of Actual Commercial Labels
|
CIVDR Automatic Renewal: Applicable if the following conditions are met:
- - Application filed before expiry date
- - Renewal Fee is paid
|
|
Transition period:
- - Class A IVDs (Blood collection tube and not included in FMC 2014-005): can be imported, distributed, exported, and manufactured without CIVDR for three* years upon effectivity. License to Operate (LTO) will serve as the document at the point of entry/bidding requirements.
- During the transition period, companies may apply for voluntary registration.
- - Class B, C and D IVDs (not included in FMC 2014-005): can be imported, distributed, exported, and manufactured without CIVDR for three years upon effectivity. License to Operate (LTO) will serve as the document at the point of entry/bidding requirements.
- During the transition period, companies may apply for voluntary registration.
*As per PFDA virtual hearing
|
Transition Period:
Implementation will be separated into two phases:
Phase 1:
- - Registration of Class B-D IVDs based on FMC 2014-005
- - Notification of all Class A IVDs
- - Notification of Class B-D IVDs not in FMC 2014-005
- - Implemented after 6 months
Phase 2:
- - Registration of All Class B-D IVDs
- - To be issued into a separate circular
|
|
COVID-19 Test Kits and Monkeypox Test Kits:
To be registered with the FDA in accordance with this AO upon effectivity.
|
COVID-19 Test Kits:
Special Certification for Imported Test Kits of COVID-19.
|
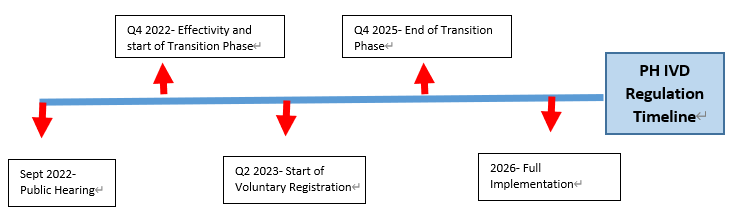
From the hearing, PFDA sets the following timeline for the latest IVD draft
References:
https://www.fda.gov.ph/announcement-notice-of-virtual-public-hearing-on-the-proposed-administrative-order-entitled-rules-and-regulations-governing-the-issuance-of-an-authorization-for-an-in-vitro-diagnostic-medical-dev/
https://www.fda.gov.ph/draft-for-comments-rules-and-regulations-governing-the-issuance-of-an-authorization-for-an-in-vitro-diagnostic-medical-device-ivd/

