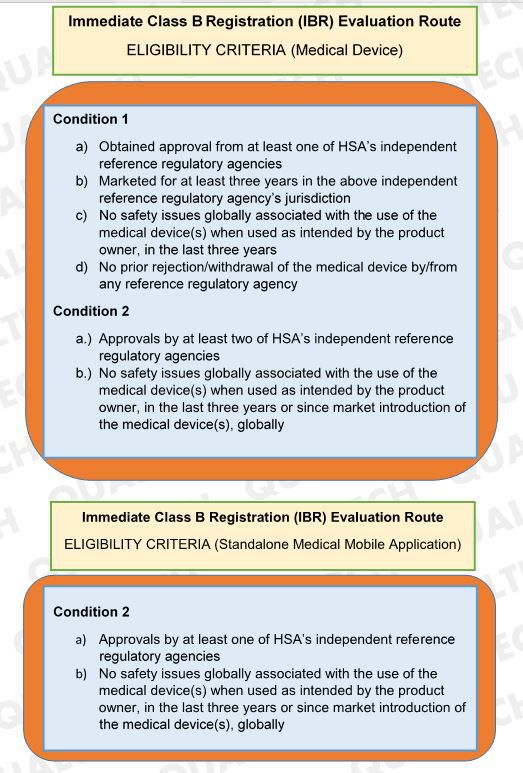
Immediate Class B Registration (IBR) Evaluation Route offers fast registration of class B devices upon submission of documents to HSA. This evaluation route is applicable to both Class B medical device and standalone medical mobile application. In the latest version of GN-15 last January 2019, HSA revised the criteria of devices to undergo IBR Evaluation Route.
In the latest revision, HSA allows Class B devices with no safety issues globally in the last three years or since the device’s market introduction to undergo IBR Evaluation if the Class B device has the required approval from HSA’s reference agencies. With this change, manufacturers of Class B products that obtain approvals in HSA’s reference agencies can avail of the IBR route if it has no safety issues recorded.
As a summary, the updated criteria for Immediate Class B Registration (IBR) Evaluation Route for medical device and standalone medical mobile application is as follows:
Summary of Eligibility Criteria for IBR Evaluation: Updated conditions for a Class B device to be processed via IBR Evaluation
References:
Reference: Health Sciences Authority- Regulatory Guidance GN-15