January 10, 2020
On December 20, 2019, the NMPA issued an update of the Exemption List from Clinical Trials in China for Medical Device (hereinafter simply referred to as the “Exemption list"), which includes "medical devices" and "in vitro diagnostic devices".
A total of 1003 medical devices and 416 IVDs are now being listed, and therefore having significantly added to the previously established four batches of exemptions.
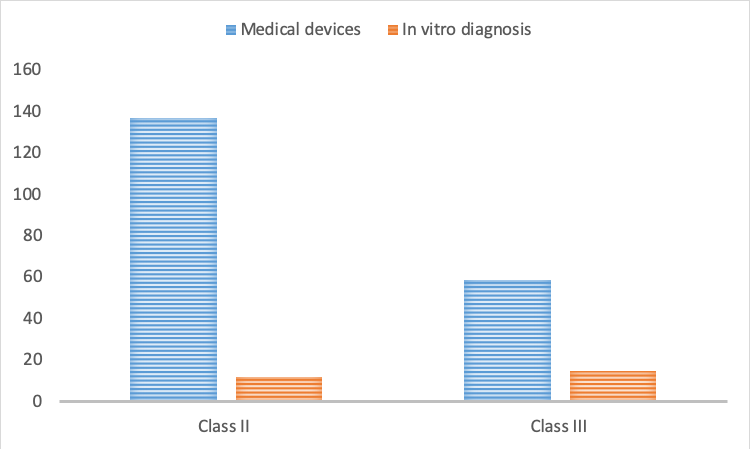
Figure: Statistic of the newly added items to the clinical exemptions list in December 2019
In detail, this new exemption list includes the category codes, product names, product descriptions, classifications, and remarks of 196 medical devices (137 of Class II and 59 of Class III), which had previously not been exempted from clinical trials in China. The exemption list covers among others “03 neurological and cardiovascular surgical instruments”, “14 infusions, nursing and protective instruments”, and “17 dental instruments”.
Moreover, there are additional 27 IVDs in the updated exemption list (12 belonging to Class II and 15 belonging to Class III). It mainly covers “the detection of narcotic drugs”, “psychotropic drugs”, and “medical toxic drugs”.
The publication of this extended exemption list is ought to further improve the registration management of medical devices for a significant number of manufacturers.
Tags:
China, Exemption list, Clinical trial
